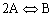

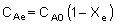
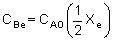
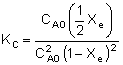
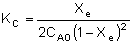
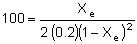
| You are given the reversible reaction: |
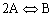 |
| which takes place in a constant volume batch reactor. The equilibrium constant, KC, for this reaction is: |
 |
| where CAe and CBe are: |
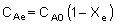
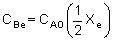 |
| Substituting for CAe and CBe gives us: |
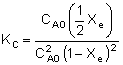 |
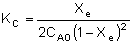 |
| Substituting known values (CA0 = 0.2 mol/dm3 and KC = 100 dm3/mol): |
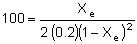 |
| Solving for the equilibrium conversion, Xe, yields: |
| Xe = 0.83 |