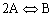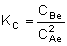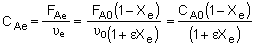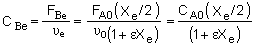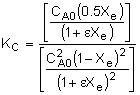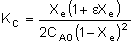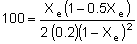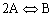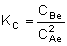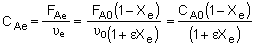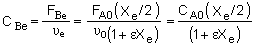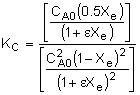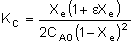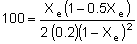| You are given the reversible reaction: |
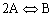 |
|
which takes place in gas phase PFR. Since gas phase reactions almost
always involve volume changes, we will have to account for volume
changes in our calculations. The equilibrium constant,
KC, for this reaction is:
|
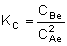
|
|
A is the limiting reactant A ⇔ B/2
|
|
where CAe and CBe are:
|
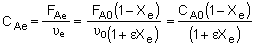
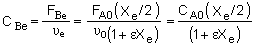
|
|
Substituting for CAe and CBe gives us:
|
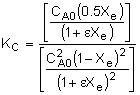
|
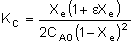 |
|
Substituting known values (CA0 = 0.2 mol/dm3
and KC = 100 dm3/mol), and realizing that:
|

|
| we end up with: |
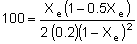 |
|
Solving for the equilibrium conversion, Xe, yields:
|
| Xe = 0.89 |