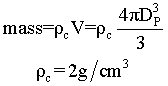
Suppose we have two samples, each with 8.38 grams of catalyst. One sample contains 10 spheres, each weighing 0.838 and one sample contains just one sphere of 8.38 g. Which sample has the greater external surface area for the molecules to enter the catalyst?
| |
|
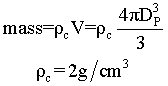 |
| 8.38 g | 8.38g |
Surface area = number of particles x surface area of one particle = n π DP2
| Large Particles | Small Particles |
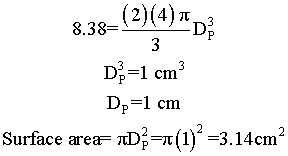 |
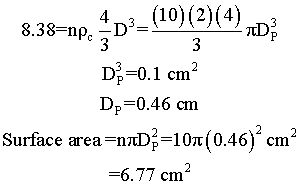 |
We see that for the same total weight of catalyst, the smaller diameter particles have a greater external surface area, and hence a grater rate constant per mass of catalyst.
Equation at bottom of page 829
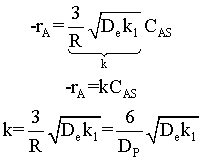
Diffusion limitations
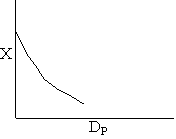
Pressure drop limitations
Laminar flow
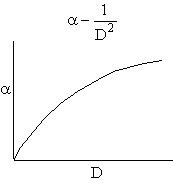
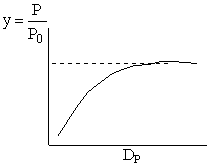
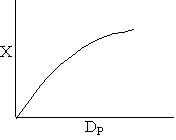
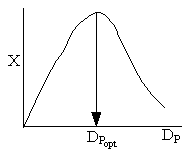
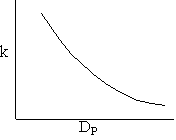
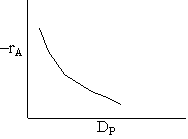
Combining diffusion limitation and pressure drop limitations as we change to particle size, we see there is an optimum particle diameter.