Suppose we want to increase the entering total
pressure. How does the pressure drop parameter, 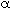 ,
vary with the entering to total pressure, Po?
[see Preface p.xx, Q5 -- Question
that probes implications and consequences. I.e., what would be the
consequence of changing the inlet pressure, Po?]
,
vary with the entering to total pressure, Po?
[see Preface p.xx, Q5 -- Question
that probes implications and consequences. I.e., what would be the
consequence of changing the inlet pressure, Po?]
a) a increases with increasing Po.
b) a decreases with increasing Po.
c) a does not change with Po.
Solution
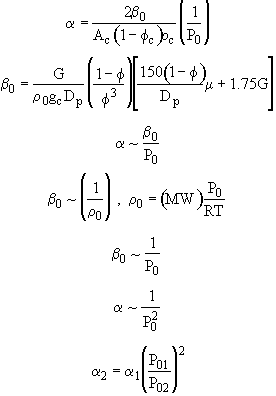
a decreases with increasing Po
We note that increasing the total pressure increases the concentration
![]()
for a fixed yao. For Pure A, yao=1.0. Therefore, the rate of reaction (A --> B) would increase.
![]()
![]()
we note we have competing effects
1) Increasing Po increases the rate initially
2) Increasing Po increases the rate at which y decreases and therefore the rate decreases
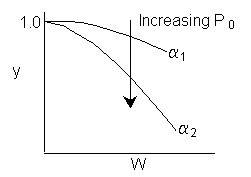
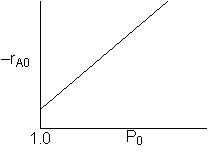
To determine which of the above effects will dominate and cause the conversion to increase or decrease will depend on a number of things such as reaction order, value of a, and other parameter values. Consequently, we would need to run the simulation. However, you will find that as Po increases, so will X, as a loose "rule of thumb".