Let’s again consider the production of ethyl benzene (click back)
![]()

The gas feed consists of 25% toluene and 75% ethylene. Write the rate of disappearance of toluene as a function of the molar flow rate. What are the coupled mole balances and rate laws?
Hint 1: What are the relative rates of reaction?
Hint 2: Write the volumetric flow rate in terms of the molar flow rates
Hint 3: Write the concentration of toluene and ethylene in terms of the molar flow rates
Hint 4: Write the rate of disappearance of A, –rA solely as a function of the molar flow rates
Hint 1.
![]()
For relative rates for measures other than conversion, the stoichiometry comes in relating the relative rates.
For this elementary reaction
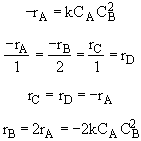
Hint 2.
Write the volumetric flow rate in terms of molar flow rates
![]()
Isothermal operation and (T = T0 and P = P0)
![]()
![]()
![]()
![]()
Hint 3.
In terms of molar flow rates
![]()
For a flow system at constant T and P
![]()
![]()
![]()
![]()
Hint 4.
In terms of molar flow rates
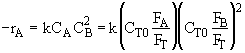
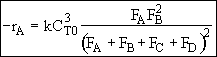
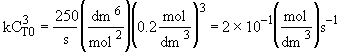
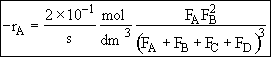
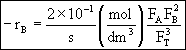
Hint 5.
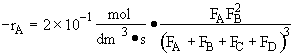

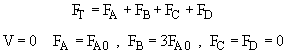
Use Polymath to solve these coupled ODEs