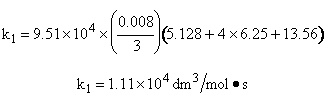The following reaction follows an elementary rate law

Initially 77% N2, 15% O2, 8% inerts are fed to a batch reactor where
80% of the equilibrium conversion (Xe = 0.02) is reached in 151 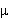 s.
What is the specific reaction rate constant k1?
s.
What is the specific reaction rate constant k1?
Additional Information
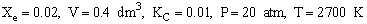
Solution
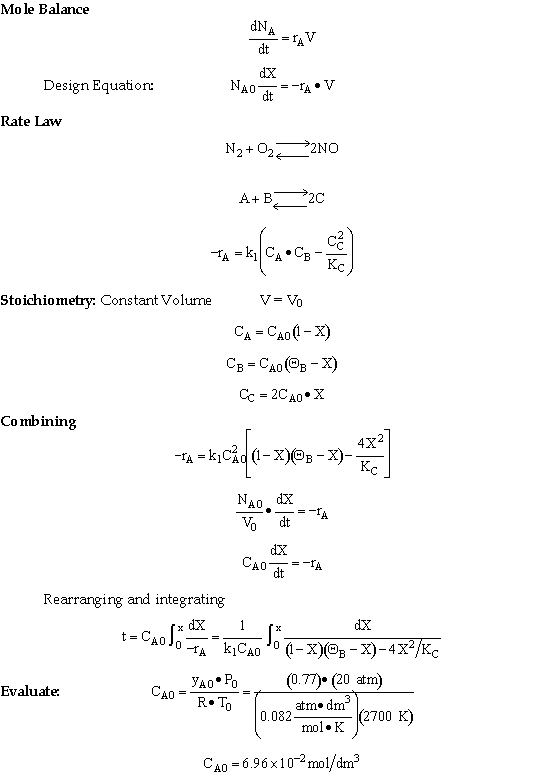
For 80% of equilibrium conversion X = 0.8 Xe = 0.016
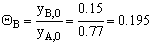
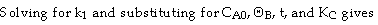
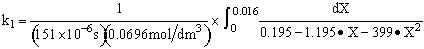
Use Simpson's three point formula to integrate with ΔX = 0.016/2 = 0.008
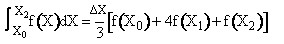
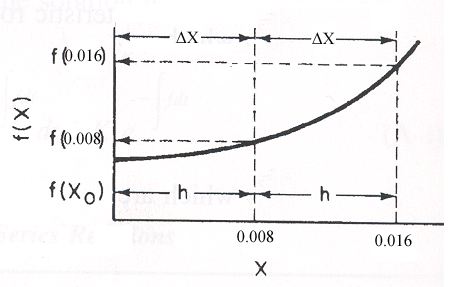
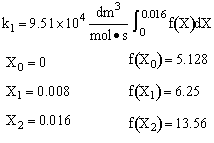
Simpson's rule: (ΔX = h = 0.008)