This section is devoted to example reaction problems. The problems were taken from the 4th Edition of Elements of Chemical Engineering by H. Scott Fogler. Both problems come from Example 8-5, the first is an adiabatic reactor and the second is a PFR with constant cooling temperature. Please note, it is assumed that the user knows how to create a flowsheet and enter process conditions, since these examples explain only the values to enter for each input window.
Jeffreys, in a treatment of the design of an acetic anhydride manufacturing facility, states that one of the key steps is the vapor-phase cracking of acetone to ketene and methane:
He states further that this reaction is first-order with respect to acetone and that the specific reaction rate can be expressed by
where k is in reciprocal seconds and T is in Kelvin. In this design, it is desired to feed 8000 kg of acetone per hour to a tubular reactor. If the reactor is adiabatic, the feed pure acetone, the inlet temperature 1035K, and the pressure 1 62 kPa (1.6 atm), a tubular reactor of what volume is required for 20% conversion?
The flowsheet consists of one inlet stream, a PFR, and one product stream. It should look like this:
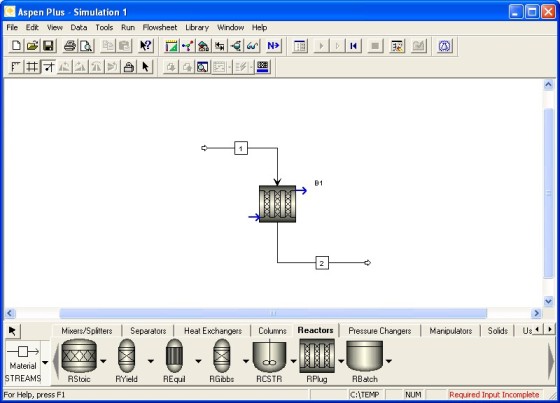
Create a flowsheet like this in ASPEN PLUSTM. If you do not know how, see Example 4-3. When the flowsheet is complete, Required Input Incomplete should appear in the lower right corner of the screen. Click the Next button. Click OK when prompted to Enter Required Data.
This section will explain what values to type in for each input window. If you do not know how to enter values, change units, or navigate through the input windows, see Example 4-3.
Setup
Properties
Stream
Blocks
Reactions - Stoichiometry
Reactions - Kinetic
Activation Energy = E = (32,444)(R)
E = (32,444)(1.987 cal/mol K) = 67999 cal/mol
Reactions - Kinetic - Driving Force
Click Next again until you are prompted to run the simulation. Click OK. When the simulation is complete, click next and choose to Display Run-Status results form. If you do not know how to interpret the results window, see Example 4-3. Otherwise, check the conversion (X = moles reacted/moles fed). Does X = 20%? If X < 20%, you must increase the length of the PFR. If X > 20%, you must decrease the length of the PFR.
In this case where length = 3 m, diam = 1m, the conversion was greater than 20%. Therefore, you need to go back to the PFR and input a smaller length. You must access the Rplug.Main window to do this. If you do not know how to reenter inputs, see Example 4-3.
This time, try a length of 2.5 m while holding the diameter constant at 1 m. When you rerun the simulation, you will find that X = 20%! Finishing up the example, the volume of the PFR with these dimensions is V = 1.96 m3.
Reference: G. V. Jeffreys, A Problem in Chemical Engineering Design: The Manufacture of Acetic Anhydride, 2nd ed. (London: Institution of Chemical Engineers, 1964).
Example 8-5 Operation of a PFR with Heat Exchanger
We again consider the vapor-phase cracking of acetone used in Example 8-5:
The reactor is to be jacketed so that a high-temperature gas stream can supply the energy necessary for this endothermic reaction (see Figure E8-5.1). Pure acetone enters the reactor at a temperature of 1035K and the temperature of the external gas in the heat exchanger is constant at 1150K. The reactor consists of a bank of one thousand 1-in. schedule 40 tubes. The overall heat-transfer coefficient is 110 J/m2-s-K. Determine the temperature profile of the gas down the length of the reactor.
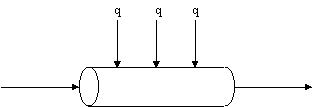
Use the same flowsheet as the adiabatic example.
Follow instrctions for Setup, Components, Properties, Stream, Reactions - Stoichiometry, reactions - Kinetic, and reactions - Kinetic - Driving Force. The only chnage from the adiabatic example is in the Block input data.
Blocks
Run the simulation. Again, adjust the length until the conversion is X = 20%. In this example, the proper length was 1.9 m with a diameter of 1 m. Thus the volume was V = 1.49 m3.
To see the temperature profile down the length of the PFR, do the following:
You should see a plot of the temperature profile that looks like this:

Back to ASPEN PLUSTM Main Page