Description
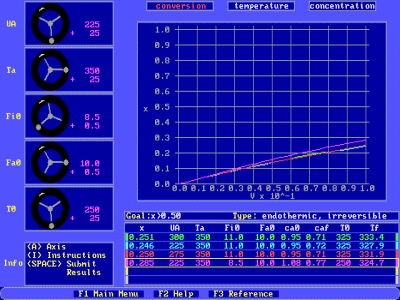
The results of the simulator may be analyzed in the form of plots of
concentration, conversion, or temperature as a function of reactor
volume.
The module may also be run in an interactive mode, in which the student
must achieve specific goals (e.g. achieve a given conversion without exceeding
a given temperature within the reactor), in order to get to the center
of the reactor complex.
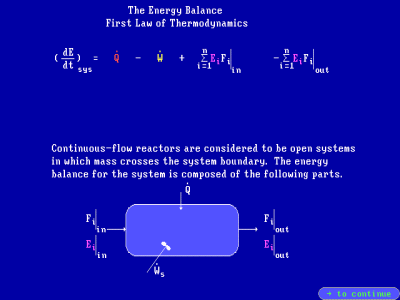
The review section includes a derivation of the energy balance equation
for a PFR:
In each case, in addition to a general statement ("increase UA"), you will be given a set of optional reactor conditions to use, in the order in which they appear in the left-hand side of the simulation screen: (UA, Ta, Fio, Fao, To). You may use these conditions if you wish, or pick your own for your investigation.
GETTING STARTED
Choose "5. individual problems" from the main menu , then choose problem 2. “Endothermic irreversible." Once the F-key bar at the bottom shows up, you may want to hit "F2" for a short description of each of the components of the simulator.
Things to keep in mind, once you are running the simulator:
• To change the step size in varying the parameters: 1(smallest
step size) 10 (largest one)
• To delete a run so its curve doesn't show up on the grap, -select
it and hit backspace.
• Not sure what keys to hit -Press I for Information.
EFFECTS OF HEAT EXCHANGE
To analyze the effect of the heat exchanger on the reaction, compare the conversion and the temperature profiles with and without heat exchange:
Set the y-axis to temperature - Choose temperature with the arrow keys.
Select your first run - Hit <Enter> until the yellow selector box
is around the UA box
Perform a run with UA equal to 0
(e.g., UA=0, Ta=300, Fio=10, Fao=10, To=300)
How does the temperature change with volume down the reactor with no heat exchange for an endothermic irreversible reaction?
Select a second run - Use the arrows to select the blue run.
Perform a new run with a higher UA
(e.g. UA=250, Ta=300, Fio=10, Fao=10, To=300)
How does the temperature change down the reactor with heat exchange for an endothermic irreversible reaction?
Set the y-axis to conversion - Press "A" for axes
How do the conversion profiles for the cases with and without heat
exchange compare?
EFFECTS OF FLOW PARAMETERS
Perform a new run with no inerts.
(e.g. UA=250, Ta=300, Fio=0, Fao=10, To=300)
Now perform a run with a higher reactant rate.
(e.g. UA=250, Ta=300, Fio=0, Fao=20, To=300)
How does the presence of inerts affect the results from the previous
question?
Perform a run with inerts
(e.g. UA=250, Ta=300, Fio=10, Fao=20, To=300).
Compare the temperature profiles for the above three cases.
APPLICATION
Given your new-found intuition, try to get the highest conversion given the limitation that the reactor temperature (at ALL positions within the reactor), must be between 250-300 K. An easily achieved value is 0.50. The highest conversion found so far is 0.711. Turn in the conditions you used (UA,Ta,Fio, Fao,To) as well as the conversion obtained, and a few sentences explaining what you learned.
SUMMARY
Write a paragraph (1/2 to 1 page) describing the effects of heat exchange on the reaction and the effects of changes in the reactant flowrate and the inert flowrate on conversion and temperature profiles for the tubular reactor. Include sketches illustrating the trends and the equations necessary to predict the results. Based on these results can you predict what would happen in an exothermic, irreversible reaction? How about reversible reactions?