Polymath
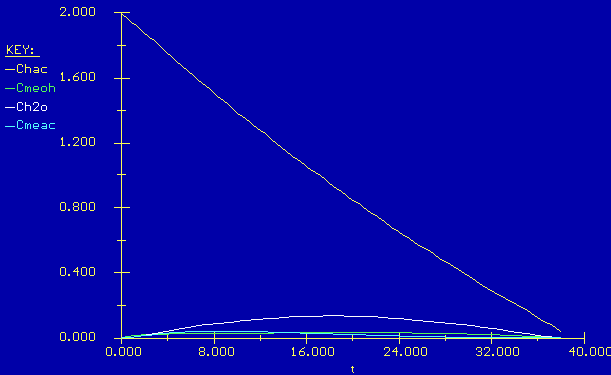
In this scenario all of the products and reactants are gone after 38
minutes. You might think this is the best conversion, but remember that
both the reactants and the products were evaporating so not all the
acetic acid reacted. In fact, the calculate d conversion is 15%.
The conversion is not the only thing that can be studied in the
different cases. Try exploring as many of the questions below to get a
better understanding of how reactive distillation and other parameters
effect the reaction.
- We have set the equations so that the reaction constant and the
equilibrium constant are dependant on temperature. What happens with the
reaction at high temperatures? Low? The boiling point of methyl acetate
is 330 K. What if the temperature is below that point? What if it is
above the boiling point of water?
- The rate at which the species evaporate are affected by the system
pressure. What happens as the pressure increases or decreases? If the
pressure in the reactor increased, what other parameters could you
change to try to bring the reaction back to i ts original state?
- What is the effect on conversion if you increase the rate at which
air is bubbled through the reactor? Sketch your answer then use Polymath
to check your hypothesis.
 Click here for clear view of graph
Click here for clear view of graph
 Click here for clear view of graph
Click here for clear view of graph