 Click here for clear view of graph
Click here for clear view of graph
These equations can now be plugged into Polymath.
Polymath
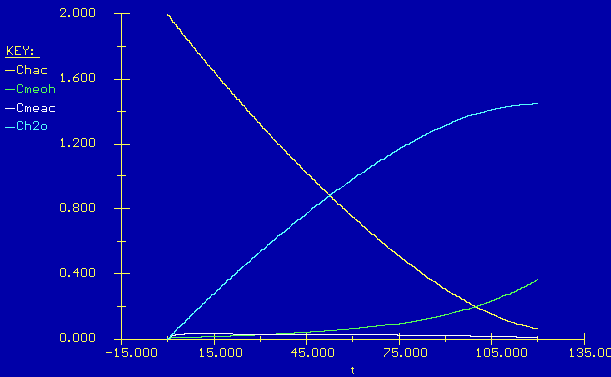
In this case only 18 moles of acetic acid is left giving a conversion of 94 %. This is much better than the case with no reactive distillation.
In real life the other products will also evaporate. We can develop similar equations for the other species to see how their evporation effects the reaction.
| Mole Balance | Mass Balance | |||
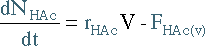 |
 |
|||
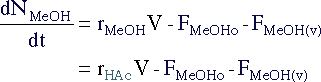 |
||||
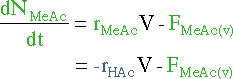 |
||||
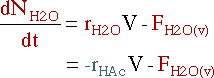 |
||||
| Rate Law | ||||
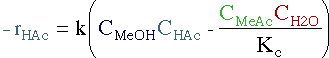 |
||||
Physical properties and vapor pressure equations for acetic acid, methanol, and water