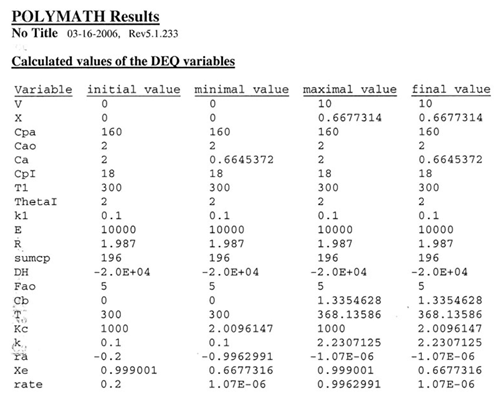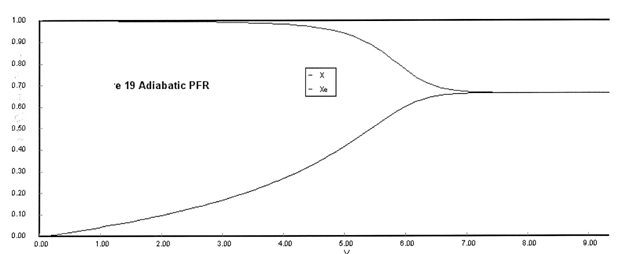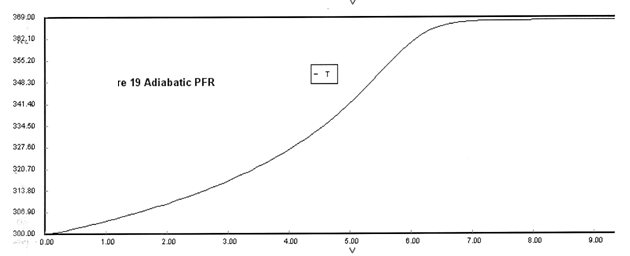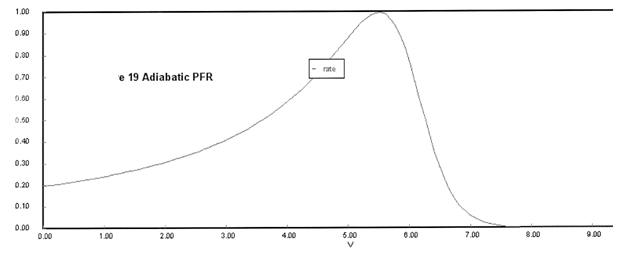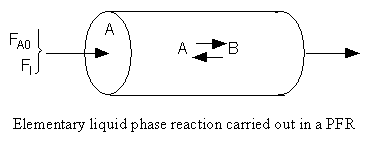

The feed consists of both inerts I and Species A with the ratio of inerts to the species A being 2 to 1.
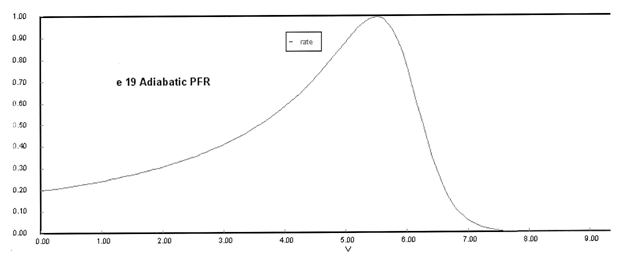
(a) Plot X, Xe, T and Rate (Rate A = –rA) as a function of V for adiabatic operation. Explain why the curves look the way they do.
Reactor
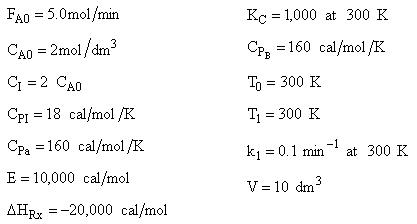
(a) Solution
| Mole Balance | ||
| (1) | 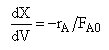 |
|
| Rate Law | ||
| (2) | 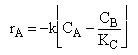 |
|
| (3) | 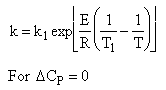 |
|
| (4) | 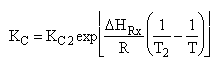 |
|
| Stoichiometry | ||
| (5) | ||
| (6) | ||
| Equilibrium | ||
| (7) | 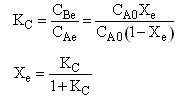 |
|
| Adiabatic Energy | ||
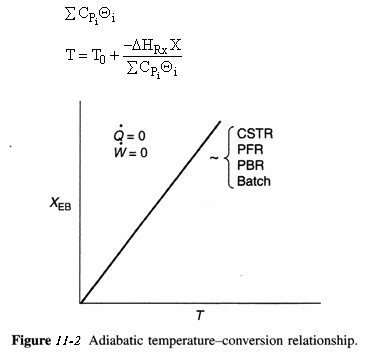 |
Adiabatic Energy Balance ΔCP = 0 and adiabatic