The irreversible liquid phase reactions
Reaction (1)
![]()
![]()
Reaction (2)
![]()
![]()

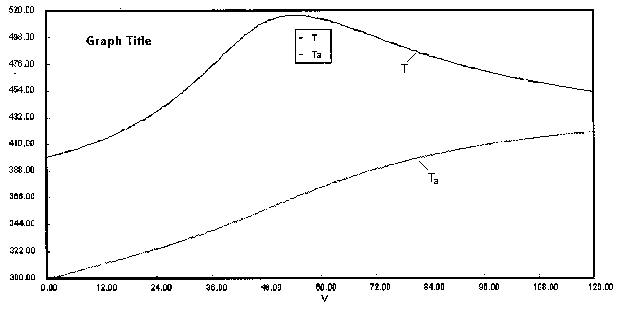
are carried out in a PFR with heat exchange. The following temperature profiles were obtained for the reaction mixture and the coolant stream.
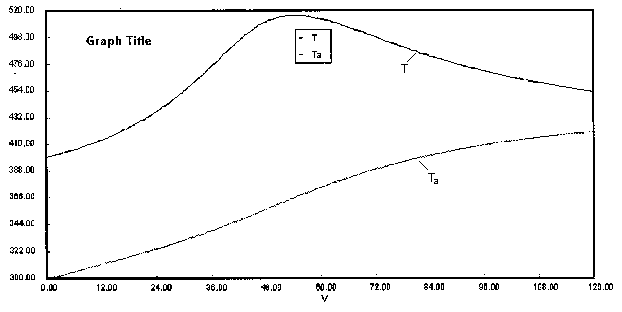
The concentrations of A, B, C, and D were measured at the point down the reactor where the liquid temperature, T, reached a maximum of 516K at 54 dm3. The concentrations at the maximum temperature were found to be CA = 0.05, CB = 0.22, CC = 0.245, and CD = 0.055 all in mol/dm3. The product of the overall heat transfer coefficient and the heat exchanger area per unit volume, Ua, is 5 cal/s•m3•K. The mole feed rate of A is 2 mol/s and the entering molar flow rate of B is 4 mol/s. The volumetric feed rate is 10 dm3/s which the molar flow rate of inerts is 0.5 mol/s. What is the activation energy for Reaction (1)? E1 =
Additional Information
![]()
![]()
![]()
Coolant flow rate = 25 g/s, and Coolant heat capacity = 20 cal/g•K
Hint 1: What is the energy balance?
Hint 2: What is Qr?
Hint 3: What are Qg1 and Qg2?

The energy balance is
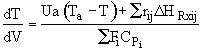
For the two reactions given here the heat of reaction for reaction (1) is in terms of A and for reaction (2) it is in terms of B
![]()
At the maximum the derivative of T wrt V is zero.
![]()
then
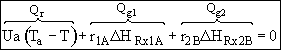
![]()
Qr
![]()
![]()
Note a quick check shows DCp = 0 for both reactions
Reaction 1
![]()
Reaction 2
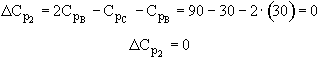
Therefore both heats of reaction are independent of temperature!!
Qg1 = r1A DHRx1A
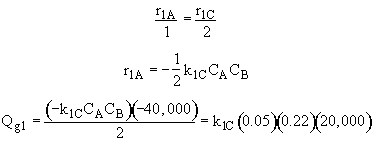
![]()
Qg2 = r2B DHRx2B
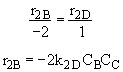
From the temperature dependence given in the problem statement
we note at 516 K that ![]()
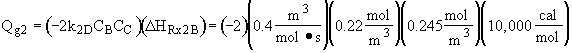
![]()
![]()
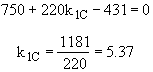
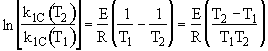
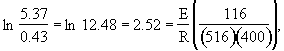
![]()
![]()