Answer
(c) PBR with Heat Exchange
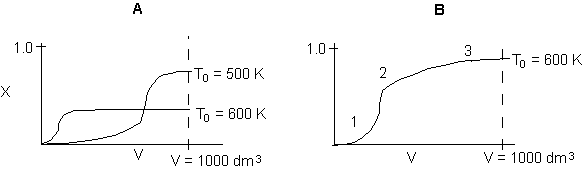
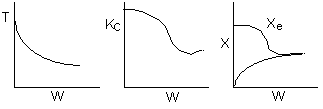
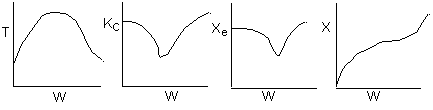
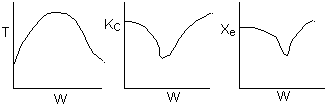
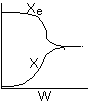
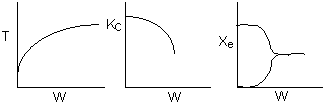
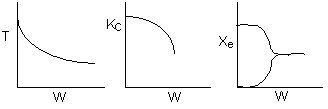
Below are the temperature, equilibrium conversion, Xe, and
conversion profiles for an exothermic, reversible reaction in a PBR.
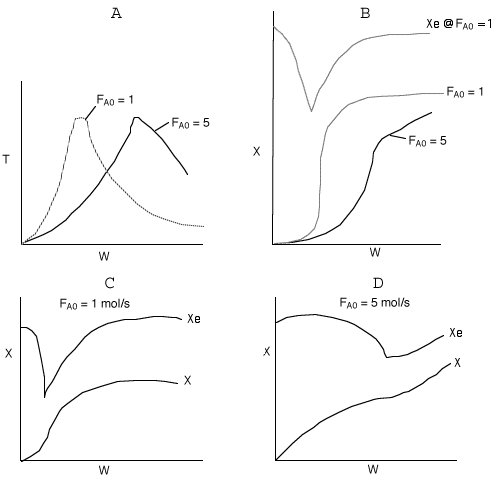
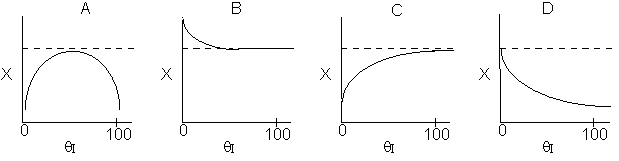
Which of the the above profiles is/are not correct?
The above profiles are for the PBR with Heat Exchange which comes directly
above from this self-test or lecture or  .
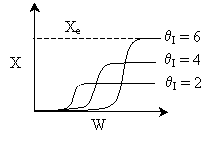
Answer
.
Answer
5.
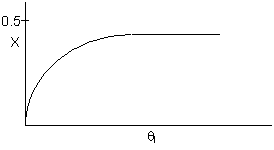
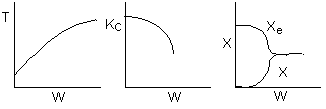
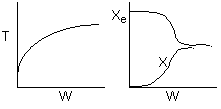
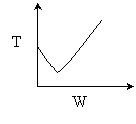
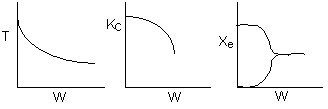
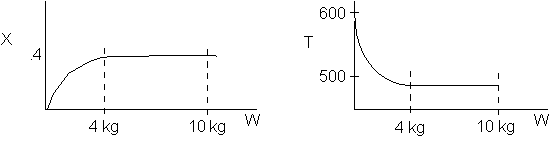
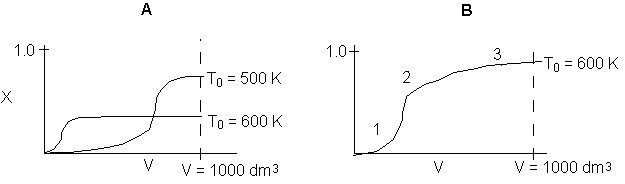
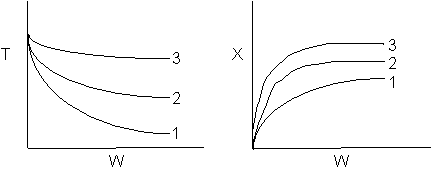
The elementary isomerization of A to B was carried out adiabatically
in a packed bed reactor. The following profiles were obtained when pure A
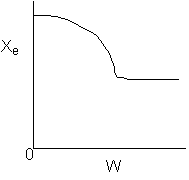
was fed to the reactor
1) The
above profiles could represent an adiabatic system where the addition of inerts
to the feed stream will increase the conversion.
2) If
the reaction is irreversible a small decrease in the flow rate will produce
a moderate increase in the conversion.
3) If
the reaction is reversible a small decrease in the flow rate will produce
a moderate increase in the conversion.
A. All the above
statements are true.
B. All the above statements
are false.
C. Statements 1 and 2 are true
D. Statements 1 and 3 are true.
E. Statements 2 and 3 are
false.
Answer
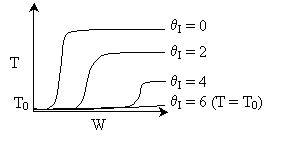
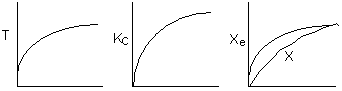
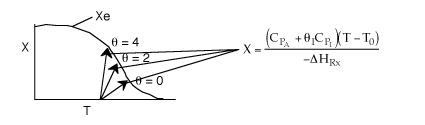
6) The elementary isomerization of A to B was carried out in a packed
bed reactor. The following profiles were obtained when pure A was fed to the
reactor
-
An increase
in the feed temperature will increase the conversion.
-
A decrease
in feed temperature will increase the conversion.
-
There could be a very very very large heat exchanger
attached to the reactor with the heat flow given by
A. All the above statements are true.
B. All the above statements
are false.
C. Statements 1 and 2 are true.
D. Statements 1 and 3 are false.
E. Statements 2 and 3 are
false.
Answer
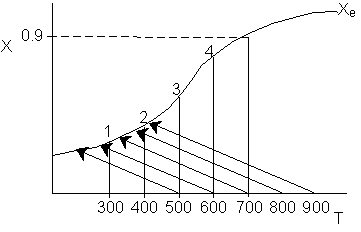
7.
The elementary, gas phase, isomerization of A to B was carried out
in a packed bed reactor. The following profiles were obtained when pure A
was fed to the reactor.
Inerts
were added to a reactor system while T0,
P0,
and n0
were kept constant. Which of the following figures the exit conversion is
as a function of the qI
is correct? i.e. qI
(qI = FI0/FA0)
Note:
? ? ? represents isothermal conversion for a reactor with 10 kg catalyst.
Answer
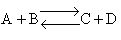
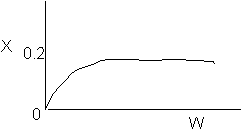
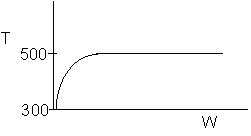
8. The elementary, gas phase reaction
takes place in a packed bed reactor. The following profiles were obtained
-
The above profiles could represent an adiabatic system
where the addition of a small to moderate amount of inerts will increase
the conversion.
-
The above profiles could represent an adiabatic system
where decreasing the flow rate will increase the conversion.
-
The above profiles could represent an adiabatic system
where if the feed temperature is increased, one cannot tell from the above
profiles whether or not the conversion will increase or decrease.
-
There could be a heat exchanger on the reactor for
which the heat flow is
A. All the above statements are true.
B. All the above statements
are false.
C. Statements 1 and 2 are true
D. Statements 2 and 4 are true.
E. Statements 2 and 3 are
false.
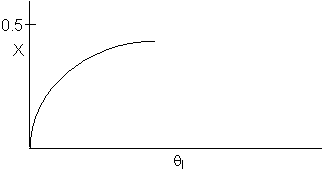
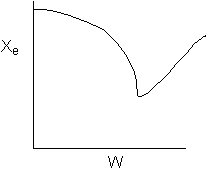
9.
The following conversion profiles were measured in two PFRs, (A and
B) for the same reaction
Which
of the following statements is true?
-
The
reaction is exothermic and reversible.
-
The
reaction is exothermic and irreversible.
-
The
reaction is endothermic and irreversible.
-
The
reaction is endothermic and reversible.
-
Cannot
tell for sure if any, some, or all the above are true.
Answer
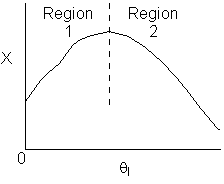
10. The exit conversion from an adiabatic PFR is shown below
as a function of θI
for constant ν0,
T0
and P0
-
The
reaction could be a second order endothermic reaction.
-
The
reaction could be a second order exothermic reaction.
-
The
reaction could be a first order exothermic reaction.
-
The
reaction could be a first order endothermic reaction.
-
The
reaction could be an irreversible zero order exothermic reaction.
-
Statements 1 and 2 are
false.
-
Statements 3 and 4 are false.
-
Statements 3 and 5 are false.
-
Statements 3 and 4 are true.
11. The exit conversion from an adiabatic PFR is shown below
as a function of θI
for constant ν0,
T0
and P0
-
The reaction could be a second
order endothermic reaction.
-
The reaction could be a second order exothermic reaction.
-
The reaction could be a first order exothermic reaction.
-
The reaction could be a first order endothermic reaction.
-
The reaction could be an irreversible zero order exothermic
reaction.
-
Both statements 1 and
2 are true.
-
Both statements 1 and 5 are true.
-
Both statements 2 and 3 are false.
-
Both statements 1 and 4 are false.
-
Both statements 4 and 5 are true.
Answer
12. The exit conversion from an adiabatic PFR is shown below as a function
of θI
for constant ν0,
T0
and P0
Which of the following statements is false?
-
The
reaction could be a second order endothermic reaction.
-
The
reaction could be a second order exothermic reaction.
-
The
reaction could be a first order exothermic reaction.
-
The
reaction could be an irreversible zero order exothermic reaction.
-
All
of the above are false.
Answer
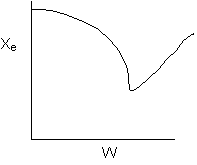
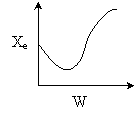
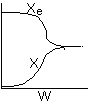
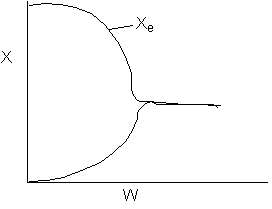
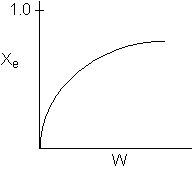
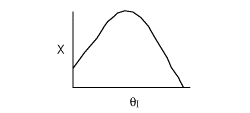
13. The equilibrium conversion is shown below as a function of catalyst
weight
-
The reaction could be first order endothermic and carried
out adiabatically.
-
The
reaction is first order endothermic and reactor is heated along the length
with Ta being constant.
-
The
reaction is second order exothermic and cooled along the length of the
reactor with Ta being constant.
-
The
reaction is second order exothermic and carried out adiabatically.
-
Both 1 and 2 are false.
-
Both 2 and 3 are false.
-
Both 3 and 4 are false.
-
Both 1 and 4 are false.
-
Both 2 and 4 are false.
Answer
14. The equilibrium conversion is shown below as a function of catalyst
weight
-
The reaction is first order endothermic and carried
out adiabatically.
-
The reaction is first order endothermic and reactor
is heated along the length with Ta
being constant.
-
The reaction is second order exothermic and cooled
along the length of the reactor with Ta being constant.
-
The reaction is second order exothermic and carried
out adiabatically.
-
Both 1 and 2 are true.
-
Both 2 and 3 are true.
-
All are true.
-
Both 3 and 4 are false.
-
Both 2 and 4 are false.
Answer
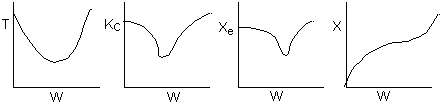
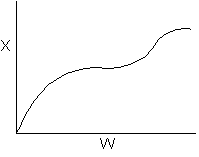
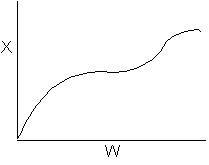
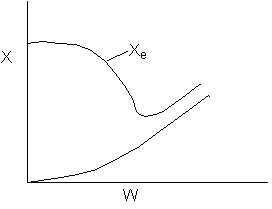
15. The equilibrium conversion is shown below as a function of catalyst
weight
Which of the following statements is false?
-
The reaction could be first order endothermic and carried
out adiabatically.
-
The
reaction could be first order endothermic and reactor is heated along
the length with Ta being constant.
-
The
reaction could be second order exothermic and cooled along the length
of the reactor with Ta being constant.
-
The
reaction could be second order exothermic and carried out adiabatically.
-
The
reaction could be first order endothermic with very high heating rate.
Answer
16. The equilibrium conversion is shown below as a function of catalyst
weight
-
The reaction could be first order endothermic and carried
out adiabatically.
-
The reaction could be could be first order endothermic
and reactor is heated along the length with Ta
being constant.
-
The reaction could be second order exothermic and cooled
along the length of the reactor with Ta
being constant.
-
The reaction could be second order exothermic and carried
out adiabatically.
-
Both 1 and 4 are false.
-
Both 1 and 3 are true.
-
Both 2 and 4 are true.
-
Both 3 and 4 are false.
-
All are true.
Answer
17. The equilibrium conversion and temperature are shown below as a function
of catalyst weight for three sets of conditions
-
The figures could correspond to an exothermic reversible
reaction with too high of a cooling rate.
-
The
figures could correspond to an exothermic irreversible reaction with too
high of a cooling rate.
-
The
figures could correspond to an endothermic reversible reaction with too
high of a heating rate.
-
The
figures could correspond to an endothermic irreversible reaction with
too high of a heating rate.
-
Both 2 and 4 are false.
-
Both 1 and 3 are false.
-
Both 3 and 4 are true.
-
Both 1 and 4 are true.
-
Both 1 and 2 are true.
Answer
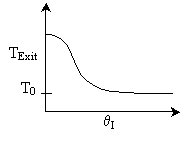
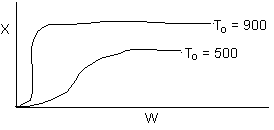
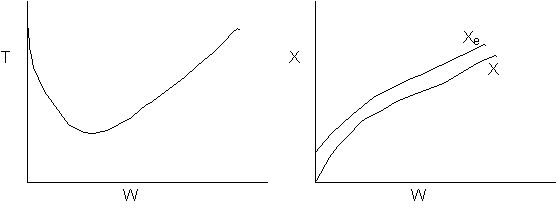
18. The conversion from an adiabatic PFR is shown below as a function of
temperature
Which of the following is false
-
90%
conversion could be achieved for a first order reaction by increasing
the inerts by a factor of 100 or greater for entering temperature of 700
K.
-
90%
conversion could be achieved for a second order reaction by adding a very
large heat exchanger with a very large area for an entering temperature
of 700 K.
-
For
a small but fixed reactor length, one is more likely to get close to the
adiabatic equilibrium conversion for an entering temperature of 500 K
than an entering temperature of 900 K.
-
The
reaction could be a second order endothermic reaction carried out with
a very large heat exchanger attached to the reactor.
-
If
the reaction is carried out adiabatically, the temperature in a CSTR will
drop more for an entering temperature of 900 K then that for an entering
temperature of 500 K.
Answer
19.
-
The
above reaction could be exothermic.
-
The
above reaction could be endothermic.
-
The
above reaction could be first order.
-
The
above reaction could be second order.
-
The
above reaction is not carried out adiabtically.
-
Both 1 and 5 are false.
-
Both 2 and 5 are false.
-
1, 2 and 3 are true.
-
3, 4 and 5 are true.
-
1, 2, 3, and 4 are true.
Answer
20.
-
The
above reaction could be carried out adiabatically.
-
The
above reaction could be exothermic.
-
The
above reaction could be endothermic.
-
The
temperature profile could go through a maximum.
-
The
temperature profile could go through a minimum.
-
1, 2, 3, and 4 are true.
-
1, 2, 5, and 5 are false.
-
1, 4 and 5 are true.
-
2, 3, 4, and 5 are true.
Answer
21.
-
The
above reaction could be adiabatic.
-
The
above reaction could be exothermic with cooling.
-
The
above reaction could be endothermic with heating.
-
The
above reaction could be second order
-
Both 1 and 2 are true.
-
Both 2 and 3 are true.
-
Both 2 and 4 are true.
-
Both 3 and 4 are true.
-
Both 1 and 4 are true.
Answer
22.
-
The
above reaction could be reversible.
-
The
above reaction could be irreversible.
-
The
above reaction could be exothermic.
-
The
above reaction could be endothermic.
-
The
above reaction could be adiabatic.
-
1, 2, 3, and 4 are true.
-
1, 3, and 4 are true.
-
1, 3, 4, and 5 are true.
-
Only 1 and 3 are true.
-
Both 2 and 5 are false.
Answer
Solutions
Problem 1
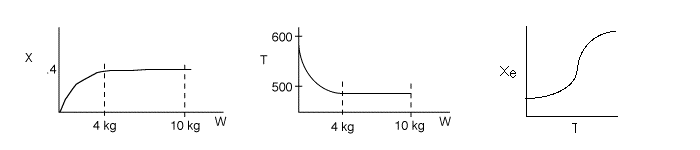
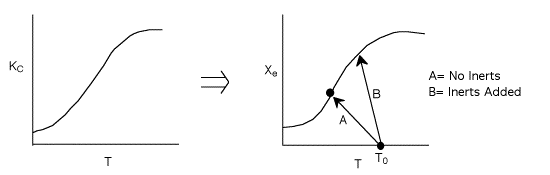
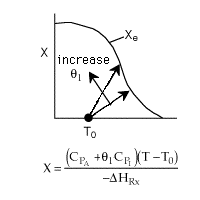
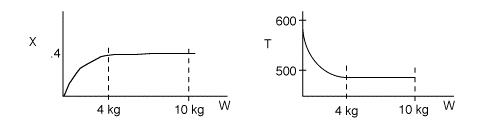
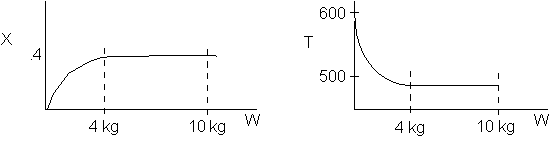
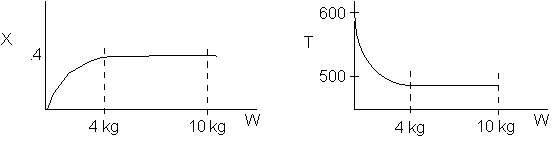
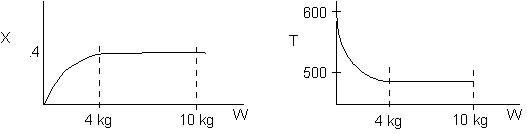
1 (a) TRUE. If it is an adiabatic system, then it has to be endothermic, because the temperature decreases and the heat of reaction is positive. Increasing inerts increases the temperature, increasing k, increasing the rate and hence increasing coversion.
|
|
Adiabatic Energy Balance 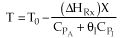 (1-1)
(1-1)
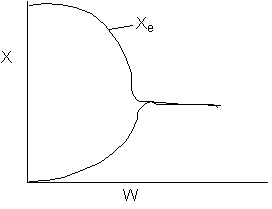
The reaction could have essentially stopped at 4 kg for one of two reasons.
A. First, the reaction could have stopped because the temperature
drops sufficiently to cause k to reach a very very small value so that
the rate is so small and reaction does not proceed farther. That
is the reaction becomes frozen.
B. The second reason the reaction could have stopped is because
it reaches equilibrium (as the temperature drops, so does Xe).
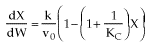 (1-5)
(1-5)
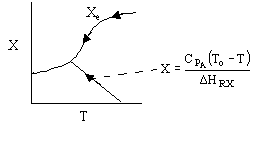
Case I: An Irreversible First-Order Endothermic Reaction
Combined Mole Balance, Rate Law, and Stoichiometry
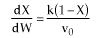 (1-2)
(1-2)
Note fixed Tτ0, if the total molar flow rate and temperature and pressure are constant (hence Cτ0) the ν0 is constant, consequently the presence of inerts does not effect this equation except by increasing
the temperature which increases k. Adding inerts to the system will cause
the temperature not to drop as much so that the rate will be faster and
the conversion greater.
In which case question (a) is true.

Case II: Reversible 1st order Endothermic Reaction
Fixed Tτ0
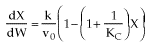
At equilibrium
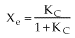 (1-6)
(1-6)
Endothermic
For no inerts (Line A)
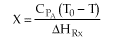 (1-7)
(1-7)
Along the energy balance line, the temperature will continue to decrease
until the reaction has reached essentially equilibrium, and the adiabatic
equilibrium conversion.
For inerts (Line B)
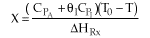 (1-8)
(1-8)
Again, increasing the amount of inerts increases the slope of the line,
the energy balance line (B) causing the temperature not to drop as much
as the case with no inerts and as shown. In addition, the equilibrium
conversion is increased.
1 (a) TRUE If the reaction is an endothermic, reversible,
and first-order then,
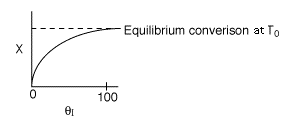
Back to Problem 1
Back to Chapter 12
1 (b) TRUE. For an irreversible reaction, decreasing
the flow rate will always increase conversion. Even if it's on the plateau,
the conversion still increases ever so slightly. The reactants spend a longer
time in the reactor.
Back to Problem 1
Back to Chapter 12
1 (c) FALSE. If the exit conversion is essentially
the equilibrium conversion (e.g., X = 0.999Xe), then
the reactants spending more time in the reactor as a result of a decrease
in the flow rate will not affect conversion, because the reaction has reached
equilibrium.
If the reaction had not reached equilibrium, then decreasing the flow rate
could increase the conversion.
Back to Problem 1
Back to Chapter 12
1 (d) TRUE. Higher entering
temperature, higher rate, higher equilibrium conversion, therefore greater
extent of reaction (greater conversion).
Back to Problem 1
Back to Chapter 12
1 (e) FALSE. If it is an endothermic, reversible
reaction, then it will have a lower equilibrium conversion at a lower temperature
(in addition, the lower temperature will cause the specific rate, k, to be
lower), resulting in a slower rate and thus smaller conversion.
Back to Problem 1
Back to Chapter 12
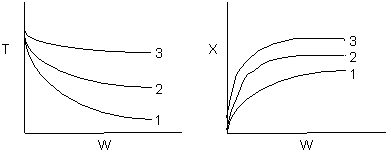
Problem 2
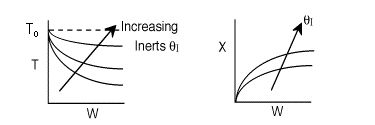
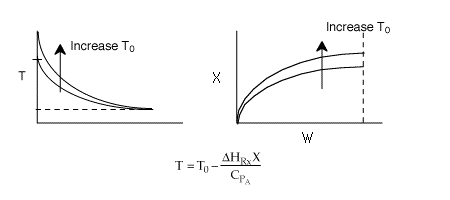
2 (a) TRUE. If it is an adiabatic system, then it has to be exothermic. Also,
because both the temperature and conversion reach a plateau, equilibrium has
been reached. Adding inerts increases the slope of the energy balance line.
 (2-1)
(2-1)
Case 1 - Rapid Reaction: Equilibrium reached even at isothermal temperature,
To.
The addition of inerts will lower the exit temperature and hence will increase
both the equilibrium conversion and the exit conversion, provided
the rate is sufficiently large to always closely approach equilibrium, even
at To, as shown in the figure below. As more and more inerts
continue to be added, the reactor approaches isothermal condition, To.
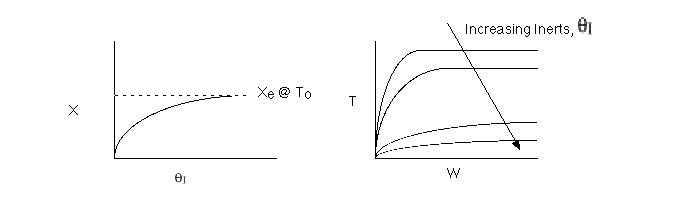
Irreversible rapid reaction, equilibrium achieved.
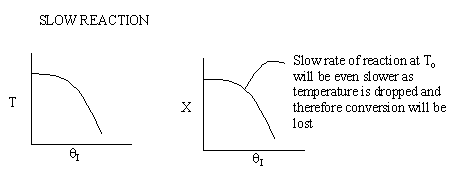
Case 2 - Slow Reaction: Equilibrium not achieved at isothermal
temperature, To.
We now consider the case when the reaction is
slow at the start (i.e. T = T0) but as it proceeds
down the reactor the temperature increases as does the rate and the conversion
until it finally takes off (see  = 0 below).
= 0 below).
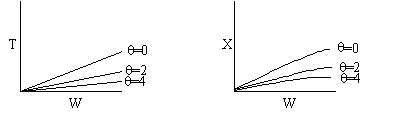
If we were to add inerts to the point that the reactor approached isothermal
conditions and the reaction rate was very small, then under these conditions
very little conversion would be achieved (as in theta=4 above).
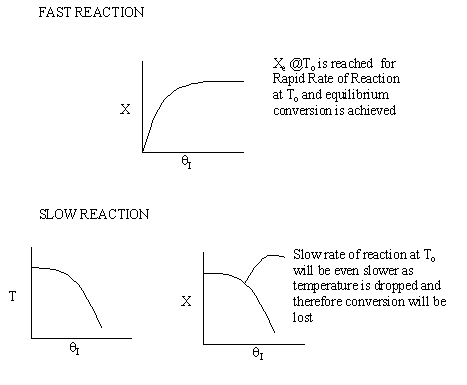
For an intermediate rate of reaction we could have
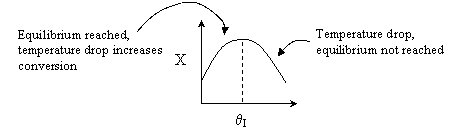
Suppose the reaction was an irreversible second-order reaction
Adding inerts will increase the temperature for endothermic reaction of
any order. Consequently, k will increase and the rate will increase with
increasing inerts, but only to a point.
Combined Mole Balance, Rate Law, and Stoichiometry


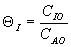
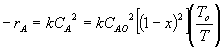 (1-3)
(1-3)
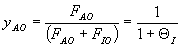
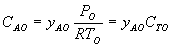
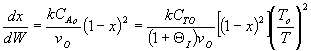 (1-4)
(1-4)
We see that at very large values of 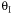 the rate
becomes very small and consequently very little conversion is achieved.
Consequently there is an optimum in the amount of inerts for a 2nd
order reaction.
the rate
becomes very small and consequently very little conversion is achieved.
Consequently there is an optimum in the amount of inerts for a 2nd
order reaction.
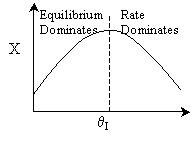
There would be an optimum value of thetaI for which the exit
conversion is a maximum. As we initially increase thetaI we decrease
the maximum temperature reached, therefore increasing Xe. However,
as we increase thetaI further, the rate decreases and we don't
approach Xe.
Back to Problem 2
Back to Chapter 12
2 (b) FALSE. Decreasing the flow rate will not change
the exit condition because it is an equilibrium condition.
Back to Problem 2
Back to Chapter 12
2 (c) FALSE. We see that for the plot of entering
temperature shown, equilibrium has already been reached in the reactor.
If feed temperature is increased, equilibrium conversion is decreased. Therefore,
one can tell whether the exit conversion will increase or decrease.
Back to Problem 2
Back to Chapter 12
Problem 3
3 (a) EXOTHERMIC. Temperature increases reaching a plateau in reactor
A at the adiabatic equilibrium conversion.
Back to Problem 3
Back to Chapter 12
3 (b) REVERSIBLE. In reactor A, the conversion reaches
a plateau (i.e.X=Xe) well below complete conversion, X=1.0, indicating
a reversible reaction.
Back to Problem 3
Back to Chapter 12
3 (c) A is adiabatic. B has a heat exchanger. As
heat is removed in Reactor B, temperature drops, shifting equilibrium to the
right to increase conversion.
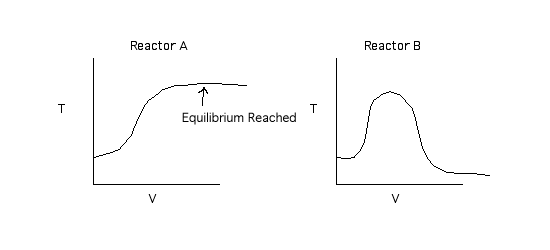
By equilibrium being reached we are saying X = 0.99Xe.
Back to Problem 3
Back to Chapter 12
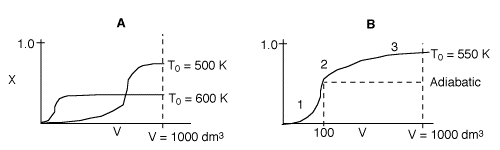
3 (d)
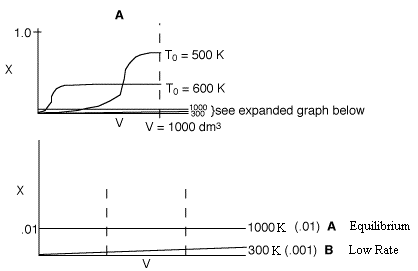
In curve A (1000 K), the equilibrium conversion is virtually reached near
the reactor entrance. However due to the high temperature, the equilibrium
conversion is very low. For curve B (300 K), this very low temperature results
in an extremely low specific reaction rate (k). Consequently, the reaction
is so slow the reaction never takes off, resulting in a very small conversion.
Therefore, there is an optimum inlet temperature. You cannot tell whether
or not conversion will increase because you do not know which side of the
optimum you are on.
Back to Problem 3
Back to Chapter 12
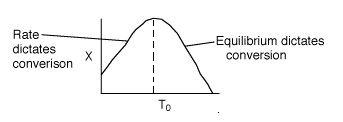
3 (e)

There is an optimum inlet temperature. Therefore you cannot say whether or
not conversion will increase or decrease if you increase T0.
It depends on which side of the optimum you are on.
Back to Problem 3
Back to Chapter 12
3 (f)
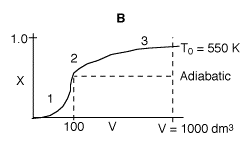
1. Very rapid rate and equilibrium is approached at 100 dm3.
If the reactor were adiabatic, then conversion would remain the same after
V=100 dm3.
2. Reactor begins to cool down shifting equilibrium to the right, conversion
slowly increases.
3. Reactants almost exhausted and low temperature results in a slow
rate and conversion levels off.
Back to Problem 3
Back to Chapter 12
Problem 4
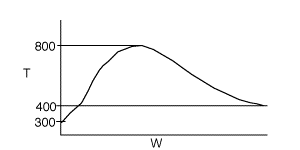
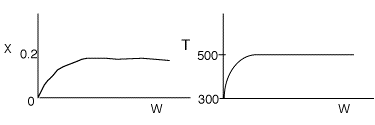
4 (a) As the temperature increases, so does the reaction rate until the
equilibrium conversion is approached at which point the reaction slows down.
As the temperature drops, Xe increases, the reaction continues
to shift to the right, thereby increasing conversion.
Back to Problem 4
Back to Chapter 12
4 (b)
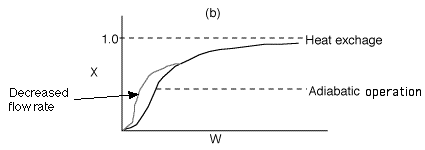
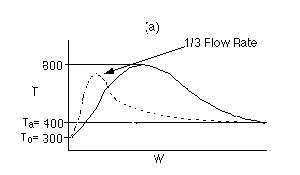
Back to Problem 4
Back to Chapter 12
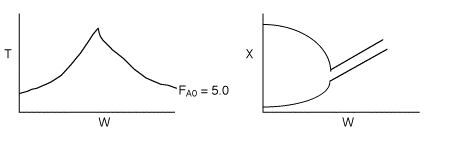
4 (c) Heat Exchange
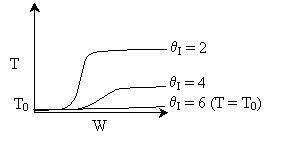
Solution: Higher flow rates will cause the temperature profile to flatten
out more near the entrance to the reactor, so (A) is correct, as is (D).
(B) and (C) are not correct because for Fao=1, the temperature begins to
decrease, Xe begins to increase, so the conversion should increase
but instead X reaches a plateau.
Back to Problem 4
Back to Chapter 12
Solution
5. The elementary isomerization
of A to B was carried out adiabatically in a packed bed reactor. The following
profiles were obtained when pure A was fed to the reactor
1) The
above profiles could represent an adiabatic system where the addition of
inerts to the feed stream will increase the conversion.
2) If
the reaction is irreversible a small decrease in the flow rate will produce
a small increase in the conversion.
3) If
the reaction is reversible a small decrease in the flow rate will produce
a small increase in the conversion.
A. All the
above statements are true.
B. All the above statements
are false.
C. Statements 1 and 2 are true
D. Statements 1 and 3 are true.
E. Statements 2 and 3 are
false.
Solution: Ans: E
1)
True. If it is an adiabatic
system then it has to be endothermic because the temperature decreases.
Increasing inerts increases the exit temperature and hence the conversion
is higher.
2)
False. The reaction has
reached a sufficiently low temperature the reaction is essentially frozen.
Thus, changing the flow rate will not produce a moderate increase the temperature
or conversion.
3)
False. If the exit condition
is an equilibrium condition, then a small change in the flow rate will not
affect the equilibrium condition. Hence, it will not change the conversion.
If the reaction is frozen, as in 2, then neither the conversion nor temperature
will increase significantly.
Back to Problem 5
Back to Chapter 12
Solution 6.
The elementary isomerization of A to B was carried out in a packed
bed reactor. The following profiles were obtained when pure A was fed to
the reactor
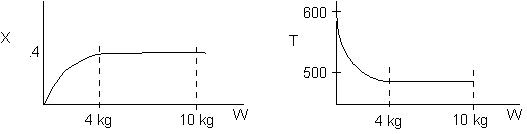
-
An increase
in the feed temperature will increase the conversion. (True)
-
A decrease
in feed temperature will increase the conversion.(False)
-
There could be a very very very large heat exchanger
attached to the reactor with the heat flow given by (False)
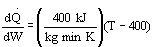
A. All the above statements are true.
B. All the above statements
are false.
C. Statements 1 and 2 are true.
D. Statements 1 and 3 are false.
E. Statements 2 and 3 are
false.
Explanation:
The reaction is endothermic because temperature drops.
1)
The higher temperature, the higher equilibrium conversion, higher
rate, therefore greater conversion.

2)
If it is an endothermic reaction then it should have smaller equilibrium
conversion at low temperature and also a smaller k resulting in a slower
rate and smaller conversion.
3)
Because final temperature is 500 K and reaction is either frozen
(i.e., the temperature is so low the reaction rate is virtually zero or
it is in equilibrium. The ambient temperature of the heat exchanger cannot
be 400.
Back to Problem 6
Back to Chapter 12
Solution
7. The elementary isomerization
of A to B was carried out in a packed bed reactor. The following profiles
were obtained when pure A was fed to the reactor
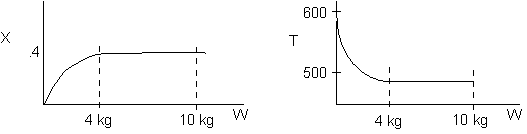
Inerts were added to a reactor system while T0,
P0,
and n0
were kept constant. Which of the following figures the exit conversion is
as a function of the qI
is correct? i.e. qI
(qI = FI0/FA0)

Solution: Ans: C.
Inerts provide sensible heat to raise temperature. The combined mole
balance rate law and stoichiometry,
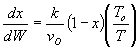
is independent of amount of inerts.
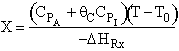
temperature will be higher because inerts supply sensible heat.
The higher temperature the higher rate and the greater the conversion.
Back
to Problem 7
Back to Chapter 12
Solution
8. The elementary, gas phase reaction
takes place in a packed bed reactor. The following profiles were
obtained
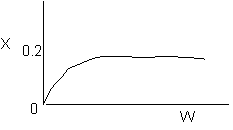
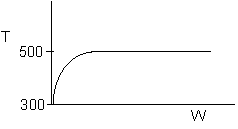
-
The above profiles could represent an adiabatic system
where the addition of a small to moderate amount of inerts will increase
the conversion. (True)
-
The above profiles could represent an adiabatic system
where decreasing the flow rate will increase the conversion. (False)
-
The above profiles could represent an adiabatic system
where if the feed temperature is increased, one cannot tell from the
above profiles whether or not the conversion will increase or decrease.
(False)
-
There could be a heat exchanger on the reactor for
which the heat flow is (True)
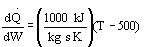
A. All the above statements are true.
B. All the above statements
are false.
C. Statements 1 and 2 are true
D. Statements 2 and 4 are true.
E. Statements 2 and 3 are
false.
Explanation:
1) TRUE
(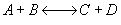 ) If it is an adiabatic
system, then it has to be exothermic. Addition of a moderate amount of inerts
will lower the exit temperature increase the equilibrium conversion and
hence will increase the conversion. If a very large amount of inerts are
added then the conversion could decrease since the combined M.B., R.L.,
and Stoich is
) If it is an adiabatic
system, then it has to be exothermic. Addition of a moderate amount of inerts
will lower the exit temperature increase the equilibrium conversion and
hence will increase the conversion. If a very large amount of inerts are
added then the conversion could decrease since the combined M.B., R.L.,
and Stoich is
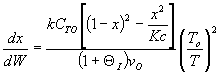
and,
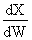 goes to zero as θI ?
?
goes to zero as θI ?
?
In
addition, k will decrease as inerts are added because the temperature is
decreased.
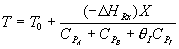
2) FALSE
Decreasing the flow rate will not change the exit condition because it is
an equilibrium condition.
3)
FALSE One can tell becuase equilibrium is achieved early in the reactor
if feed temperature is increased, equilibrium conversion is decreased.
4)
TRUE The ambient temperature is 500 K, the same as the final equilibrium
temperature. Consequently if the reaction is endothermic, heating the reactor
will increase the rate, Xe and X.
Answer:
E
Back to Problem 8
Back to Chapter 12
Solution 9.
The following conversion profiles were measured in two PFRs, (A and
B) for the same reaction
Which
of the following statements is true?
-
TRUE
The reaction is exothermic and reversible.
-
FALSE
The reaction is exothermic and irreversible (equilibrium is reached).
-
FALSE
The reaction is endothermic and irreversible (temperature increases,
exothermic).
-
FALSE
The reaction is endothermic and reversible (temperature increases, exothermic).
-
Cannot
tell for sure if any, some, or all the above are true.
Solution:
A is true, at high temperature equilibrium achieved early in reactor.
Back to Problem 9
Back to Chapter 12
Solution 10.
The exit conversion from an adiabatic PFR is shown below as a function
of θI
for constant ν0,
T0
and P0
-
The
reaction could be a second order endothermic reaction. False.
-
The
reaction could be a second order exothermic reaction.
False.
-
The
reaction could be a first order exothermic reaction. True.
-
The
reaction could be a first order endothermic reaction.
True.
-
The
reaction could be an irreversible zero order exothermic reaction.
False.
-
Statements 1 and 2
are false.
-
Statements 3 and 4 are false.
-
Statements 3 and 5 are false.
-
Statements 3 and 4 are true.
Explanation:
1) FALSE
(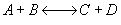 )
)
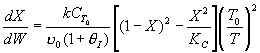 , as θI
becomes very large, X decreases.
, as θI
becomes very large, X decreases.
2) FALSE
(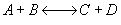 )
)
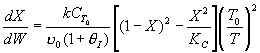 , as θI
becomes very large, X decreases.
, as θI
becomes very large, X decreases.
3) TRUE
(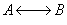 )
)
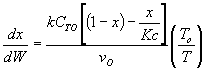 ,
,  does not appear in this combined mole
balance, rate law, and stoichiometry, but as
does not appear in this combined mole
balance, rate law, and stoichiometry, but as  increases,
temperature decreases, k decreases, and Kc increases
as does Xe.
increases,
temperature decreases, k decreases, and Kc increases
as does Xe.
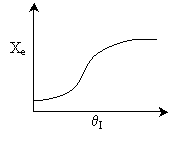
Case I: k very very large. Equilibrium is always achieved in
the column.
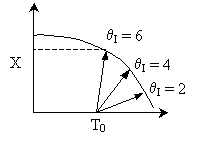
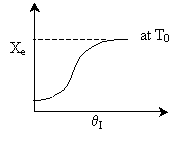
Even at T = T0 down the reactor, equilibrium
is reached. So it could be a first order exothermic reaction. TRUE
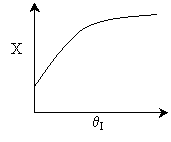
Case II: k is moderate. Equilibrium is not always achieved.
k is so low at T0, reaction never really takes
off. In this case the answer would be FALSE. But because Case
I is possible, the overall answer is TRUE.
4) TRUE (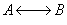 )
)
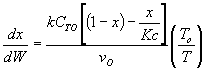 , as &thetaI
increases, so does T, Xe
and X.
, as &thetaI
increases, so does T, Xe
and X.
5) FALSE (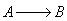 )
)
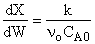 ,
,
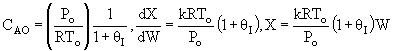 conversion increases as &thetaI
increases.
conversion increases as &thetaI
increases.
Case I: E is very small so that decreasing the temperature by
adding inerts does not change k very much at all. Thus,
Case II: Large E, decreasing the temperature by adding inerts
decreases T and k at the exit. Smaller k, smaller conversion.
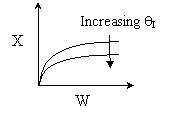
Back to Problem 10
Back to Chapter 12
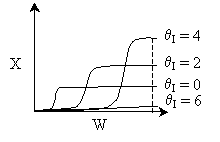
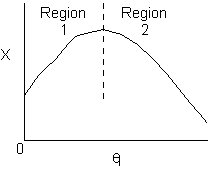
Solution 11. The exit conversion from an adiabatic PFR is shown below
as a function of θI
for constant ν0,
T0
and P0
- The reaction could be a second order endothermic reaction.
True.
- The reaction could be a second order exothermic reaction. True.
- The reaction could be a first order exothermic reaction. True.
- The reaction could be a first order endothermic reaction. False.
- The reaction could be an irreversible zero order exothermic reaction.
False.
- Both statements 1 and 2 are true.
- Both statements 1 and 5 are true.
- Both statements 2 and 3 are false.
- Both statements 1 and 4 are false.
- Both statements 4 and 5 are true.
Explanation:
answer 6
True. In region 1 increasing qI
increases temperature and X. In region 2,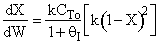 . Large
. Large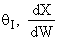 decrease, therefore X decreases.
decrease, therefore X decreases.
2) True. In region 1, adding inserts could decrease temperatures
and increase equilibrium conversion. In region 2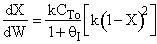 .
Large
.
Large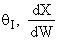 decrease, therefore
X decreases.
decrease, therefore
X decreases.
3) True. 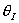 does not appear in this combined mole balance, rate law, and stoichiometry,
but as
does not appear in this combined mole balance, rate law, and stoichiometry,
but as 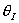 increases, temperature decreases, k decreases,
and Kc increases as does Xe.
increases, temperature decreases, k decreases,
and Kc increases as does Xe.
3) True. 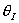 does not appear in this combined mole balance, rate law, and stoichiometry,
but as
does not appear in this combined mole balance, rate law, and stoichiometry,
but as 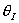 increases, temperature decreases, k
decreases, and Kc increases as does Xe.
increases, temperature decreases, k
decreases, and Kc increases as does Xe.
Case I: k very very large. Equilibrium is always achieved
in the column.
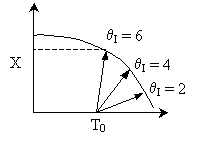




Even at T = T0 down the reactor, equilibrium
is reached.
Case II: k is moderate. Equilibrium is not always achieved.





k is so low at T0, reaction never really takes
off.
4) False.
For first order
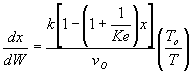 independent of inserts except
in k and KC. Increase θI
increase T, k, and X up until we reach isothermal conditions then X will
be independent of &thetaI.
independent of inserts except
in k and KC. Increase θI
increase T, k, and X up until we reach isothermal conditions then X will
be independent of &thetaI.
5) False.
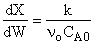 ,
,
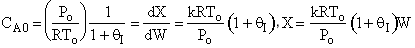 conversion increases as θI
increases.
conversion increases as θI
increases.
Case I: E is very small so that decreasing the temperature by
adding inerts does not change k very much at all. Thus,

Case II: Large E, decreasing the temperature by adding inerts
decreases T and k at the exit. Smaller k, smaller conversion.



Back to Problem 11
Back to Chapter 12
Solution 12. The exit conversion from an adiabatic PFR is shown below as a function
of θI
for constant constant ν0,
T0
and P0
Which of the following statements is false?
- The reaction could be a second order endothermic reaction. True
- The reaction could be a second order exothermic reaction. True
- The reaction could be a first order exothermic reaction. True
- The reaction could be an irreversible zero order exothermic reaction.
True
- All of the above are false.
A) True if DHRX is very small such that temperature does
not change upon adding inerts and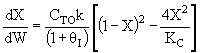 ,
then for large Kc, X could decrease as qI increases.
,
then for large Kc, X could decrease as qI increases.
B) True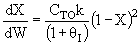 adding
inerts could decrease temperature and therefore k and X.
adding
inerts could decrease temperature and therefore k and X.
C) True.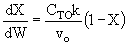 , increase
inerts, could decrease T, k and X
, increase
inerts, could decrease T, k and X
D) False.
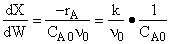
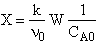
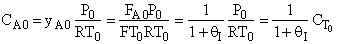
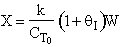
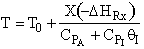
Increases
with qI
Back to Problem 12
Back to Chapter 12
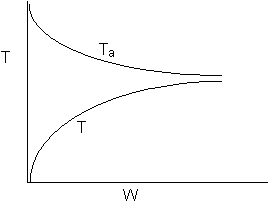
Solution 13. The equilibrium conversion is shown below as a function of catalyst
weight
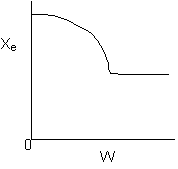
- The reaction could be first order endothermic and carried out adiabatically.
True.
- The reaction is first order endothermic and reactor is heated along
the length with Ta being constant. False.
- The reaction is second order exothermic and cooled along the length
of the reactor with Ta being constant.
False.
- The reaction is second order exothermic and carried out adiabatically.
True.
- Both 1 and 2 are false.
-
Both 2 and 3 are false.
- Both 3 and 4 are false.
- Both 1 and 4 are false.
- Both 2 and 4 are false.
Explanation:
1) As temperature
drops so does KC
and Xe.
However X continues to increase until Xe is reached.
2) Flat profile rules
out heating or cooling.
3) If cooling, temperature
would decrease. Xe would increase at some point if there
were heating or cooling.
4)
Back to
Problem 13
Back to Chapter 12
Solution 14. The equilibrium conversion is shown below as a function of catalyst
weight
- The reaction could be first order endothermic and carried out adiabatically.
False? No reason for Xe
to increase if adiabatic.
- The reaction could be first order endothermic and reactor is heated
along the length with Ta
being constant. True.
- The reaction could be second order exothermic and cooled along the
length of the reactor with Ta
being constant. True.
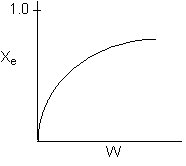
- The reaction could be second order exothermic and carried out adiabatically.
False ? No reason for Xe
to increase if adiabatic.
- Both 1 and 2 are true.
- Both 2 and 3 are true.
- All are true.
- Both 3 and 4 are false.
- Both 2 and 4 are false.
Answer: B
Back to Problem 14
Back to Chapter 12
Solution 15. The equilibrium conversion is shown below as a function of catalyst
weight
Which
of the following statements is false?
A. False. The reaction
could be first order endothermic and carried out adiabatically. Temperature
will drop.
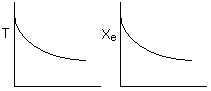
B. False. The reaction could be first order endothermic and reactor is
heated along the length with Ta being constant. If 1st order endothermic,
then
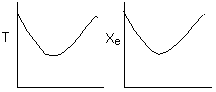
C.
False. The reaction could be second order exothermic and
cooled along the length of the reactor with Ta being constant. If 2nd order
exothermic, then
D. False. The reaction
could be second order exothermic and carried out adiabatically. If 2nd
order exothermic and adiabatic, then
E. True. The
reaction could be first order endothermic with very high heating rate.
If heating rate such that temperature always increases, then
Back
to Problem 15
Back to Chapter 12
Solution 16. The equilibrium conversion is shown below as a function of catalyst
weight
1) False. The reaction
could be first order endothermic and carried out adiabatically. If adiabatic,
then
2) True. The
reaction could be first order endothermic and reactor is heated along
the length with Ta
being constant.
3) True. The
reaction could be second order exothermic and cooled along the length
of the reactor with Ta
being constant.
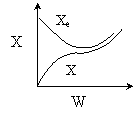
4) False. The reaction could be second order exothermic
and carried out adiabatically.
- Both 1 and 4 are false.
- Both 1 and 3 are true.
- Both 2 and 4 are true.
- Both 3 and 4 are false.
- All are true.
Answer A
Back to Problem 16
Back to Chapter 12
Solution 17. The equilibrium conversion and temperature are shown below as a function
of catalyst weight for three sets of conditions
- The figures could correspond to an exothermic reversible reaction
with too high of a cooling rate. True. k(T) smaller
\
X smaller
- The figures could correspond to an exothermic irreversible reaction
with too high of a cooling rate. True. k(T) smaller \
X smaller
- The figures could correspond to an endothermic reversible reaction
with a high heating rate. True.
T higher, k larger, Xe larger, heating rate of (3) > (2) > (1).
For endothermic reactions
- The figures could correspond to an endothermic irreversible reaction
with a high heating rate. True.
For endothermic reactions X always increases as T increases.
- Both 2 and 4 are false.
- Both 1 and 3 are false.
- Both 3 and 4 are true.
-
Both 1 and 4 are true.
- All are true.
Answer: E
Back to Problem 17
Back to Chapter 12
Solution 18. The conversion from an adiabatic PFR is shown below as a function of
temperature
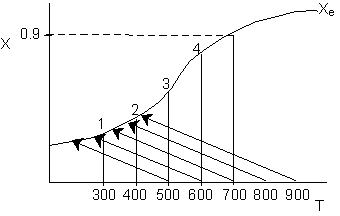
Which
of the following is false
- 90% conversion could be achieved for a first order reaction by increasing
the inerts by a factor of 100 or greater for entering temperature
of 700 K. True.
- 90% conversion could be achieved for a second order reaction by
adding a very large heat exchanger with a very large area for an entering
temperature of 700 K. True.
- For a small but fixed reactor length, one is more likely to get
close to the adiabatic equilibrium conversion for an entering temperature
of 500 K than an entering temperature of 900 K. False.
- The reaction could be a second order endothermic reaction carried
out with a very large heat exchanger attached to the reactor. False.
- If the reaction is carried out adiabatically, the temperature in
a CSTR will drop more for an entering temperature of 900 K then that
for an entering temperature of 500 K. True.
Explanation:
1.
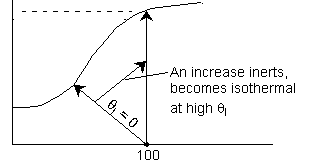
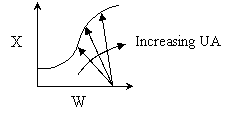
2. Eventually becomes
isothermal as the product of UA continually increases. Same as (1) above.
3. High
T0, higher rate, equilibrium conversion
reached near entrance to reactor.
4. False. If very
large heat exchanger, the energy balance term will be essentially vertical.
5.
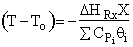 . Higher T0, higher rate, higher X greater temperature drop.
. Higher T0, higher rate, higher X greater temperature drop.
Back to Problem 18
Back to Chapter 12
Solution 19.
- The above reaction could be exothermic.
- The above reaction could be endothermic.
- The above reaction could be first order.
- The above reaction could be second order.
- The above reaction is not carried out adiabatically.
- Both 1 and 5 are false.
- Both 2 and 5 are false.
- 1, 2 and 3 are true.
- 3, 4 and 5 are true.
- 1, 2, 3, and 4 are true.
Answer: D
Explanation:
1). True.
2) True.
3) True.
4). True.
5) False ? if
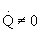 then
then
 or
or
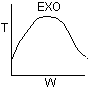
Back to Problem 19
Back to Chapter 12
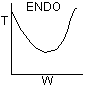
Solution 20.
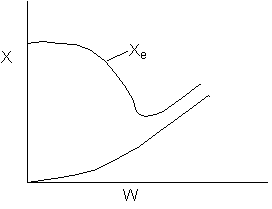
- The above reaction could be carried out adiabatically.
- The above reaction could be exothermic.
- The above reaction could be endothermic.
- The temperature profile could go through a maximum.
- The temperature profile could go through a minimum.
- 1, 2, 3, and 4 are true.
- 1, 2, 5, and 5 are false.
- 1, 4 and 5 are true.
- 2, 3, 4, and 5 are true.
Explanation:
1). False. Xe cannot increase if it is adiabatic.
2)
3) True.
3) True.
4). True. If exothermic
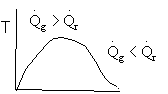
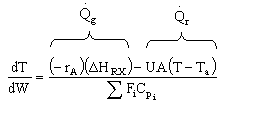
5) True. If endothermic
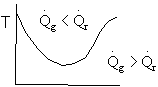 Here
both
Here
both
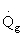 and
and
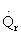 are negative
are negative
Back to Problem 20
Back to Chapter 12
Solution 21.
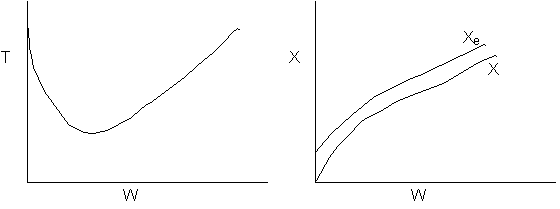
- The above reaction could be adiabatic.
- The above reaction could be exothermic with cooling.
- The above reaction could be endothermic with heating.
- The above reaction could be second order
- Both 1 and 2 are true.
- Both 2 and 3 are true.
- Both 2 and 4 are true.
- Both 3 and 4 are true.
- Both 1 and 4 are true.
Answer: D
Explanation:
- False. Xe could not increase if adiabatic.
- False. Xe would follow slope temperature curve.
- True.
- True.
Back to Problem 21
Back to Chapter 12
Solution 22.
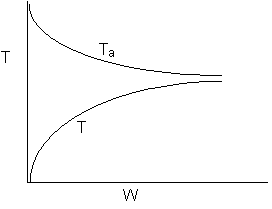
- The above reaction could be reversible.
- The above reaction could be irreversible.
- The above reaction could be exothermic.
- The above reaction could be endothermic.
- The above reaction could be adiabatic.
- 1, 2, 3, and 4 are true.
- 1, 3, and 4 are true.
- 1, 3, 4, and 5 are true.
- Only 1 and 3 are true.
- Both 2 and 5 are false.
Explanation:
- True: Heat Added to Reactor at high rate
- True: Heat Added to Reactor at high rate
- True: Heat Added to Reactor at high rate
- True: Heat Added to Reactor at high rate
- False. Ta ambient temperature changes.
Back to Problem 22
Back to Chapter 12
![]() .
.
![]()

![]()


![]() .
.
![]()
![]()
![]()
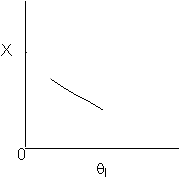


![]() (1-1)
(1-1)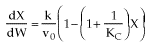 (1-5)
(1-5)

![]() (1-2)
(1-2)


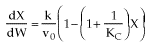
![]() (1-6)
(1-6)

![]() (1-7)
(1-7)
![]() (1-8)
(1-8)
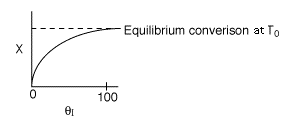


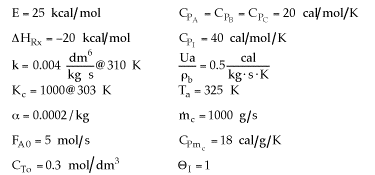
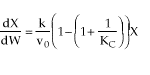
 (2-1)
(2-1)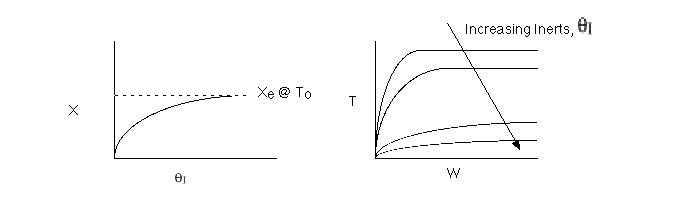
![]() = 0 below).
= 0 below).
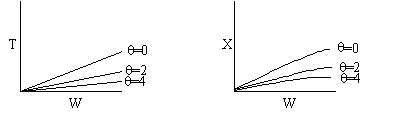
![]()


![]()
![]()
![]()
![]() (1-3)
(1-3)![]()
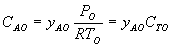
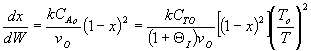 (1-4)
(1-4)
![]() the rate
becomes very small and consequently very little conversion is achieved.
Consequently there is an optimum in the amount of inerts for a 2nd
order reaction.
the rate
becomes very small and consequently very little conversion is achieved.
Consequently there is an optimum in the amount of inerts for a 2nd
order reaction.
![]()
![]()
![]()






![]()


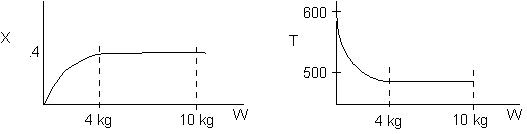
![]()



![]()
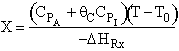
![]()


![]()
![]() ) If it is an adiabatic
system, then it has to be exothermic. Addition of a moderate amount of inerts
will lower the exit temperature increase the equilibrium conversion and
hence will increase the conversion. If a very large amount of inerts are
added then the conversion could decrease since the combined M.B., R.L.,
and Stoich is
) If it is an adiabatic
system, then it has to be exothermic. Addition of a moderate amount of inerts
will lower the exit temperature increase the equilibrium conversion and
hence will increase the conversion. If a very large amount of inerts are
added then the conversion could decrease since the combined M.B., R.L.,
and Stoich is
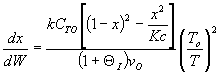
![]() goes to zero as θI ?
?
goes to zero as θI ?
?![]()


![]() )
)
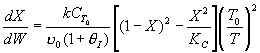 , as θI
becomes very large, X decreases.
, as θI
becomes very large, X decreases.![]() )
)
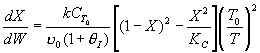 , as θI
becomes very large, X decreases.
, as θI
becomes very large, X decreases.![]() )
)
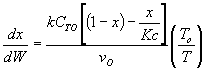 ,
, ![]() does not appear in this combined mole
balance, rate law, and stoichiometry, but as
does not appear in this combined mole
balance, rate law, and stoichiometry, but as ![]() increases,
temperature decreases, k decreases, and Kc increases
as does Xe.
increases,
temperature decreases, k decreases, and Kc increases
as does Xe. 









![]() )
)
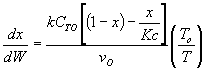 , as &thetaI
increases, so does T, Xe
and X.
, as &thetaI
increases, so does T, Xe
and X.![]() )
)
![]() ,
,
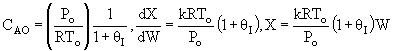 conversion increases as &thetaI
increases.
conversion increases as &thetaI
increases.




![]() . Large
. Large![]() decrease, therefore X decreases.
decrease, therefore X decreases.
![]() .
Large
.
Large![]() decrease, therefore
X decreases.
decrease, therefore
X decreases.
![]() does not appear in this combined mole balance, rate law, and stoichiometry,
but as
does not appear in this combined mole balance, rate law, and stoichiometry,
but as ![]() increases, temperature decreases, k decreases,
and Kc increases as does Xe.
increases, temperature decreases, k decreases,
and Kc increases as does Xe.
![]() does not appear in this combined mole balance, rate law, and stoichiometry,
but as
does not appear in this combined mole balance, rate law, and stoichiometry,
but as ![]() increases, temperature decreases, k
decreases, and Kc increases as does Xe.
increases, temperature decreases, k
decreases, and Kc increases as does Xe.
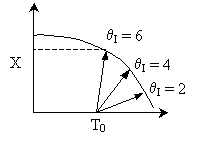









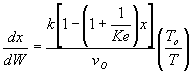 independent of inserts except
in k and KC. Increase θI
increase T, k, and X up until we reach isothermal conditions then X will
be independent of &thetaI.
independent of inserts except
in k and KC. Increase θI
increase T, k, and X up until we reach isothermal conditions then X will
be independent of &thetaI.![]() ,
,
![]() conversion increases as θI
increases.
conversion increases as θI
increases.



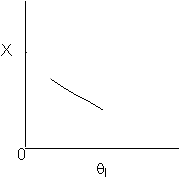
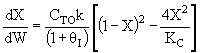 ,
then for large Kc, X could decrease as qI increases.
,
then for large Kc, X could decrease as qI increases.
![]() adding
inerts could decrease temperature and therefore k and X.
adding
inerts could decrease temperature and therefore k and X.
![]() , increase
inerts, could decrease T, k and X
, increase
inerts, could decrease T, k and X
![]()
![]()
![]()
![]()
![]()























![]() . Higher T0, higher rate, higher X greater temperature drop.
. Higher T0, higher rate, higher X greater temperature drop.


![]() then
then or
or





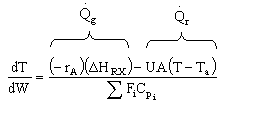
 Here
both
Here
both
![]() and
and
![]() are negative
are negative

