








A.


B.

| Applications of the PFR/PBR User Friendly Energy Balance Equations | top |
NOTE: The PFR and PBR formulas are very similar.
 |
 |
|
 |
 |
|
If we include pressure drop: |
||
C. |
||
Note: the pressure drop will be greater for exothermic adiabatic reactions than it will be for isothermal reactions |
||
 |
||
Balance on Heat Exchanger Coolant Solve simultaneously using an ODE solver (Polymath/MatLab). If Ta is not constant, then we must add an additional energy balance on the coolant fluid: |
||
| Co-Current Flow | 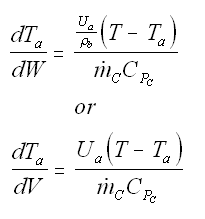 | |
| Counter-Current Flow |
with Ta = Tao at W = 0 |
|
For an exothermic reaction: with counter current heat exchange
|
|
A Trial and Error procedure for counter current flow problems is required to find exit conversion and temperature.
* All chapter references are for the 1st Edition of the text Essentials of Chemical Reaction Engineering .