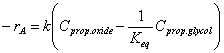
EFFECTS OF DIFFERENT PARAMETERS ON A REVERSIBLE REACTION
So far, we have only examined the effects of different parameters on an irreversible reaction. Letís turn our exothermic irreversible reaction reversible by adding a fictive equilibrium constant to the reaction rate expression and see how this affects the result.
With water still in excess, the reaction rate then takes the form:
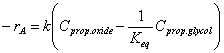
Neglecting the difference in heat of capacity between reactants and product the temperature dependence of Keq is expressed by:
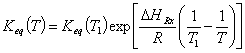
To see some effects of different parameters on this fictive reversible reaction, press continue.