|
Myoglobin stores oxygen in your muscles. The ligand binds to an iron atom that is coordinated to a heme porphyrin (purple). The ligand is also brightly colored in the above structure, but you can't see it! How does it get out? |
Ultrafast Protein Dynamics Biological systems such as proteins, DNA, membranes, cells, and organelles present spectacular challenges to our understanding of chemical dynamics and structure. Most protein molecules have reasonably well-defined structures to the extent that they can be characterized by X-ray diffraction and NMR spectroscopy. These structures, though, must necessarily respond to their environments which can vary from solid to water to oily membranes (or all three). Globular proteins, for example, are nearly solid density, and yet to function they must often be flexible. Essentially, many biological molecules are distinguished by the very fact that they are not neatly categorized as solids or liquids, but are rather something in between. In order to push towards a detailed microscopic description of these hard-to-classify biological systems, we are developing an array of optical spectroscopy tools that will complement the already commonly used X-ray and NMR techniques. We rely heavily on state-of-the-art femtosecond (1 fs = 10-15 sec) laser pulses and through various nonlinear optical processes we are able to generate significantly intense pulses at any wavelength from the ultraviolet to the infrared. Our main approach is to take advantage of the rich chemical specificity and well-developed intuition of vibrational transitions in order to track the course of chemical events. Vibrational transitions can be excited both “resonantly” through infrared absorption, or “nonresonantly” through Raman scattering, and the information content relates directly to the displacement of atoms, thus limiting our reliance on complicated electronic transitions. Our experimental approach is based on the workhorse of multidimensional spectroscopy to find out how different motions within a molecule, or between molecules are coupled together. NMR has benefited from these advances for a number of years, and now we are excited to be able to push them into the optical domain, thus giving us access to unparalleled sub-picosecond temporal resolution. These tools will offer us a new and exciting perspective on the complex dynamics of biological molecules. |
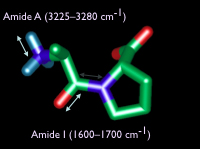
The amide bands of proteins are very sensitive reporters of secondary structure. This proline-alanine didpeptide shows the principal motions of the amide I (1600-1700 cm-1) and the amide A (3225-3280 cm-1) bands. Most of the amide I mode is carbonyl strech with a little carbon-nitrogen. The amide A is mostly nitrogen-hydrogen motion. (C = green, N = blue, O = red, H = cyan) |
|
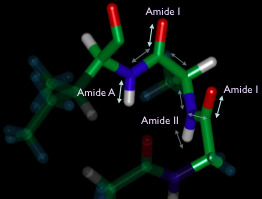
The three different amide bands (amide II is mostly N-H bending) provide nearly full coverage of the dynamics of the protein backbone. Changes to the coupling can be followed by 2D spectroscopy following a triggering event (such as a temperature jump or a ligand dissociation).
|