
Simply put, an infrared spectra is created by changes in the dipole moments in molecules. Dipole moments can be created when the bonds in a molecule shift or bend. These shifts in the molecule are created when the molecule is bombarded with energy in the form of Infrared light. The output is a record of how much of the light is absorbed at each frequency. A stretch or bend that is recorded is said to be IR active. If it is not recorded it is IR inactive.
Four factors tend to produce fewer experimental peaks than would be expected:
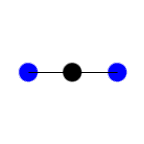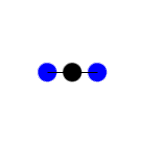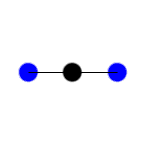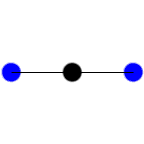
In this example of carbon dioxide symmetric and asymmetric stretching, left and right respectively, which stretching action is IR active?


For more help on understanding the stretching and bending that produces peaks in a spectrum, the Department of Chemistry at Michigan State University has developed a site with links from spectra to movies of the vibrations. Very cool.