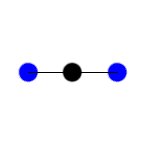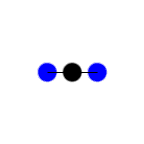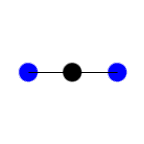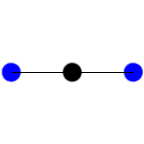
 Symmetric
Stretching
Symmetric
Stretching Symmetric
Stretching
Symmetric
StretchingWhat needs to be thought of when deciding if a bond is going to be
infrared active?
1. What, if any, is the dipole moment of the molecule?
2. What happens to the partial dipole moments of the molecule and how
does that effect the total dipole moment?
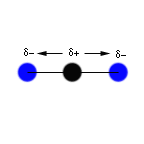
The carbon dioxide molecule has no initial dipole moment because the
partial dipole moments cancel each other out. As the oxygens move
toward and away from the carbon at the same speed and in the same
direction, the overall dipole moment change is zero. Therefore, this
stretching action does not have the change in dipole moment necessary
to register in an IR spectrum.