LADH Exhibits H/D Exchange
The RTP of alcohol dehydrogenase from equine liver (LADH)
has been studied by several research groups. LADH is a dimeric enzyme
of 40 kDa per subunit with two tryptophan residues per subunit.
Only W314, located at the subunit interface, is phosphorescent at room
temperature. The RTP lifetime of W314 is multi-exponential, and has
been shown to be sensitive to ligand binding and to subunit interactions
in LADH.
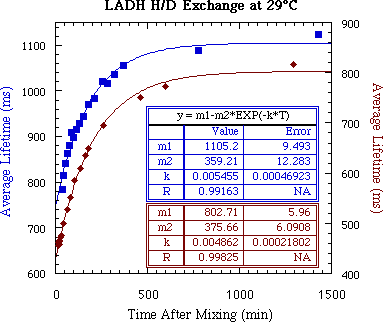
Figure 4: Exchange kinetics for two separate H/D exchange
experiments with LADH plus 1 mM ADPR at 29°C exchanging
from 10 mM pH 7.4 HEPES to 10 mM
pD 7.7 HEPES. The points shown are the average of the
multiple lifetimes in each decay. The difference in the maximum
lifetime is due to variations in the residual O2 concentration,
which strongly quenches the phosphorescent triplet state.
Escherichia coli Alkaline Phosphatase

