Static and Dynamic Heterogeneity
Single molecule experiments have been useful in detecting new
properties of enzymes such as static heterogeneity (differences between
individual molecules in the native state) and dynamic heterogeneity
(slow conformational transitions that regulate catalysis).
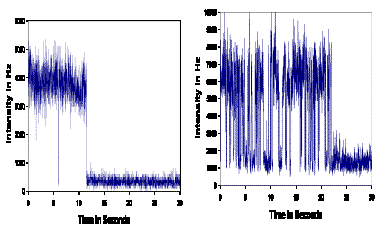
Individual molecules of PHBH show a wide variation in rates of
transition between the in and out conformation even after the enzyme
has been purified to homogeneity by FPLC. The out to in transition
(light to dark) in particular is very heterogeneous with some molecules
rapidly going back and forth from out to in and some slowly or not at
all.
The cause of the heterogeneity is unknown at this point. An
interesting possibility is that differences in the reaction rates
reflect the position of the loop around the conserved residues Proline
292 and Proline 293. The negative charge on the 4 position of the
p-hydroxybenzoate substrate repels the dipole on Proline 293 and the
resulting force is transmitted through the rigid di-proline segment to
the rest of the backbone of the protein. This force is eventually
responsible for the movement of the flavin to the out position.
Mutating Serine 293 is known to drastically increase the flexibility of
the enzyme. A dynamic heterogeneity may arise by differences in the
position of the loop either altering the rate of entry of water into
the active site or by altering the speed in which the negative charge
of hydroxide is transmitted into movement of the flavin.
Main Page
People
Current Research
Facilities
Publications
Links

