Generating and Using a Calibration Graph
Beer's Law
In the example of a calibration graph for this experiment, you are plotting absorbance vs. concentration, as opposed to an absorbance spectrum where you are plotting absorbance vs. wavelength. But how are wavelength and concentration related to absorbance? They are all related in through the Beer-Lambert Law.
The main absorbance equation is the Beer-Lambert Law which is:


Where A is the absorbance
ε is the molar absorptivity constant. This is different for every chemical, at every wavelength
l is the path length, the distance of solution that the light has to travel through
c is the concentration of the solution
The absorbance is based primarily on those three factors
Molar Absorptivity Constant
The Molar Absorptivity Constant is specific for every single solution, and at every wavelength. When you are taking an absorbance spectrum, and measuring the absorbance at different wavelengths, this is the only factor that is changing, as the concentration of the solution remains the same, and so does the pathlength. The path length of each vial is the same, and the concentration of each of these solutions is the same, yet the solutions are all different colors and will therefore absorb differently. The only difference to change the absorbance, is the Molar Absorptivity Constant.

Questions to think about:
- Would the absorbance change if you use a different solvent?
- Which of the three factors would be affected by the change in solvent?
Path Length
The path length also affects absorbance. With a longer path length, the light has to travel through more solution, and can hit more molecules, and be absorbed. This would make the aborbance increase and make the solution appear darker.
The pictures below show how solutions appear when you look through a longer pathlength.
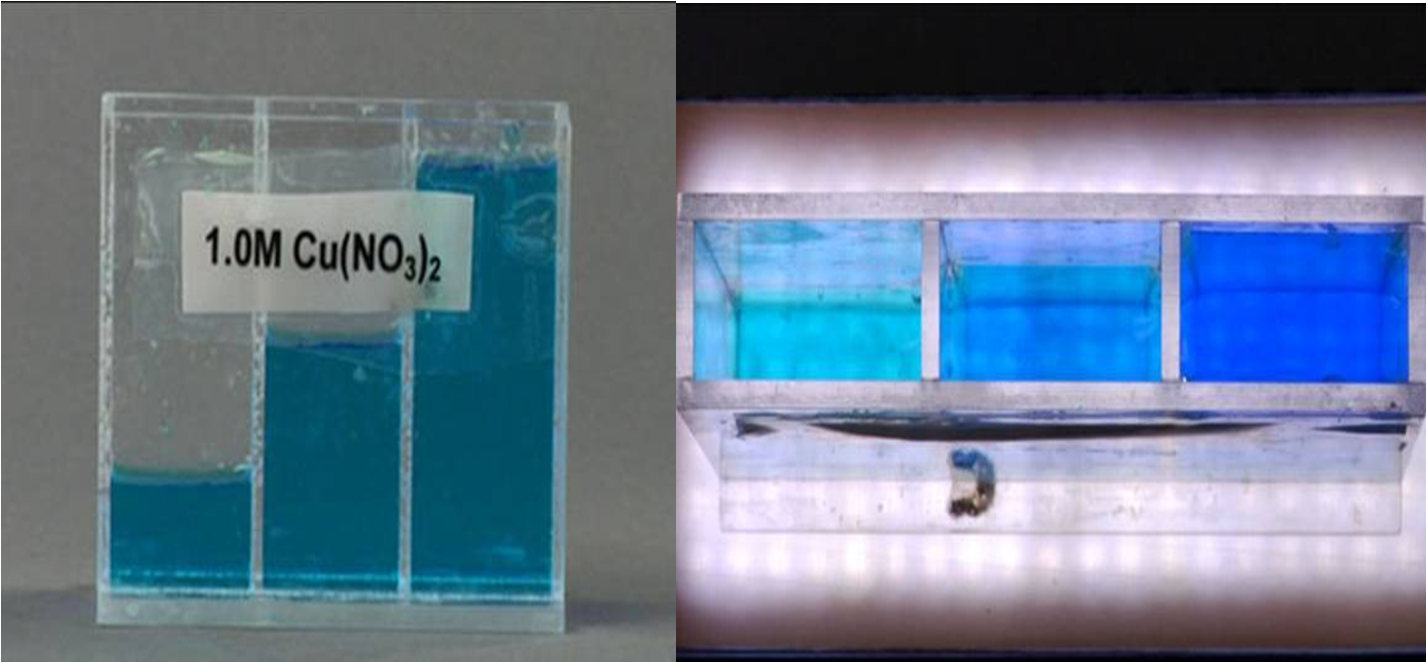
The image to the left shows a container with three compartments. Each compartment has the same solution but filled to different levels. When looking through the solutions horizontally, they all appear the same color because the path length is the same for all three. However, when you look at the image to the right, where the view is from the top, you can see that the solutions get darker as you move from left to right.
This can also be seen in absorbance spectra, where as the pathlength is increased, the absorbance is also increased.
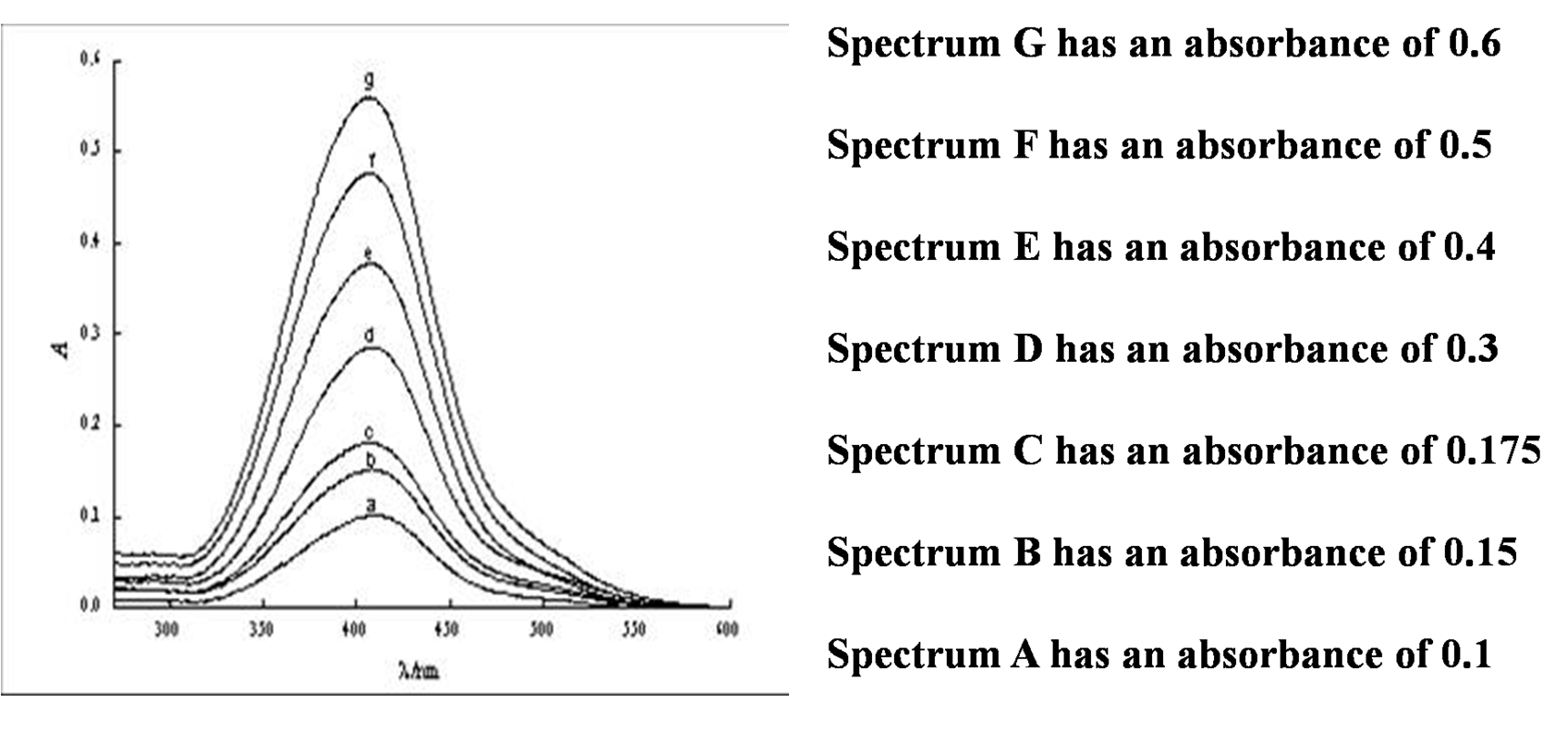
Using this information, and the spectra in the figure, see how the path length would effect the absorbance in the following questions.
Some important information is that path length is rarely ever changed away from 1.0 cm.
Concentration
The last component of Beer's Law, is concentration. Concentration effects the absorbance very similarly to path length. If the concentration of solution is increased, then there are more molecules for the light to hit when it passes through.
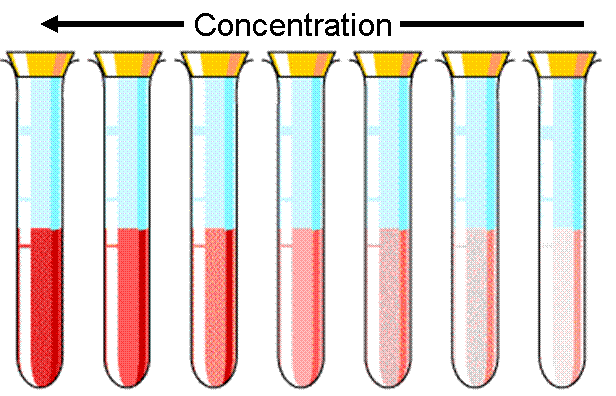
As the concentration increases, there are more molecules in the solution, and more light is blocked. This causes the solution to get darker because less light can get through.