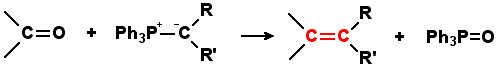|
Leading
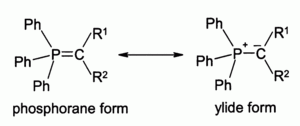
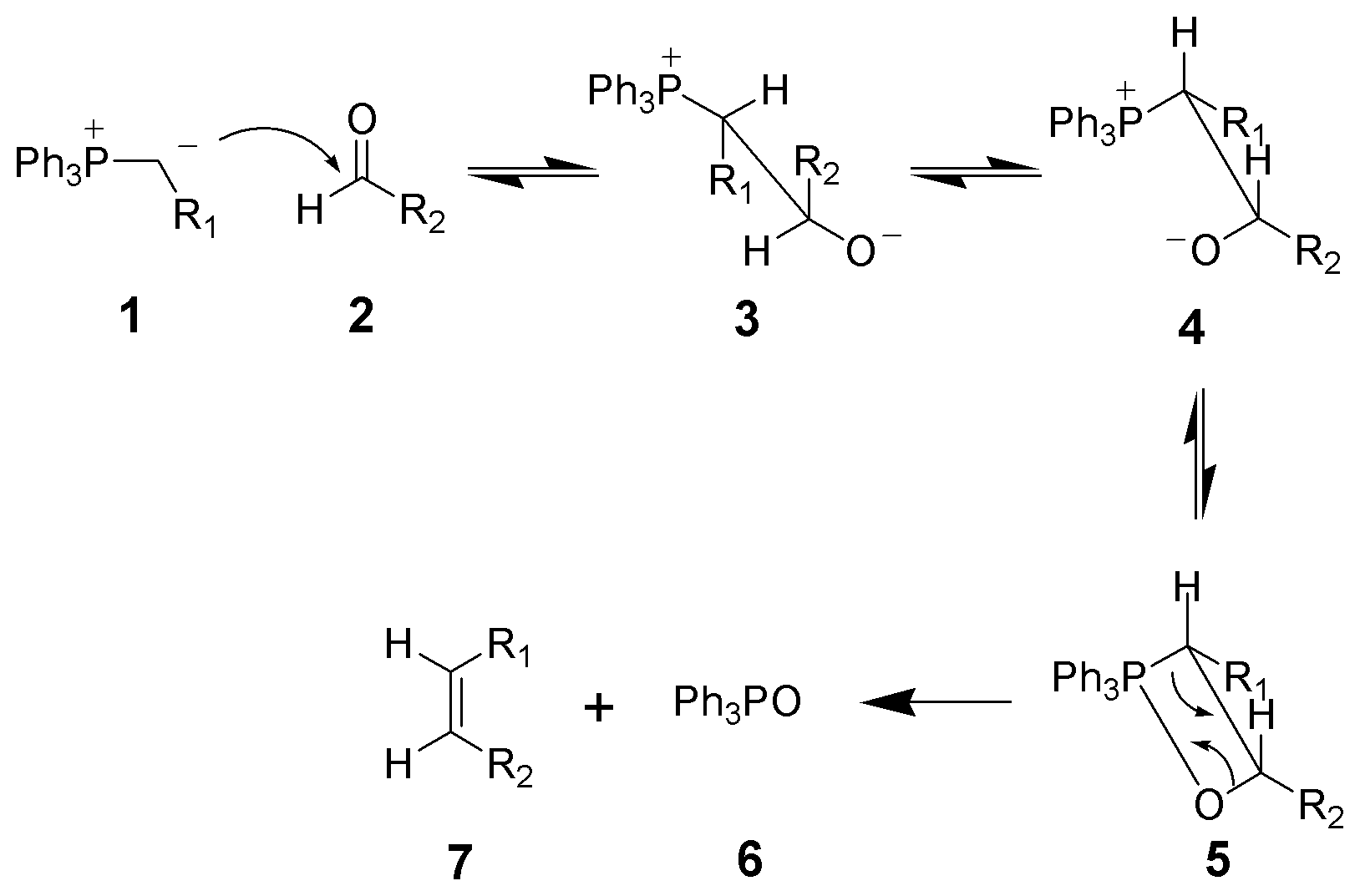
Question What is Wittig Olefination? The Wittig-olefination reaction is commonly used to convert an aldehyde or a ketone to an alkene through the use of a triphenyl phosphonium ylide (Wittig reagent). A ylide is a neutral molecule with positive and negative charges on adjoining atoms. Triphenyl phosphonium ylide is the significant resonance contributor of the phosphorane form:
Schlosser modification of the Wittig-olefination: To produce the E-alkene, threo betaine is used. Threo betaine is a diastereomer of the erythro betaine utlized in typical Wittig-olefination reactions. Threo betaine is produced by adding phenyllithium to the erythro betaine at low temperature to create a betaine ylide. Afterwards, hydrochloric acid is added to create threo betaine. |