|
References for "Divergent Enantioselective Synthesis of (-)-Galanthamine and (-)-Morphine" Trost, B.M.; Tang, W.; Toste, F.D. J. Am. Chem. Soc. 2005, 127, 14785-14803. Papers
that relate to Step 1 (Wittig Olefination): “Generation
of phosphoranes derived from phosphites. A new class of phosphorus
ylides
leading to high E selectivity with semi-stabilizing groups in Wittig
Olefinations” Aggarwal, V.K.; Fulton, J.R.; Sheldon, C.G.; De Vicente, J.
J. Am.
Chem. Soc. 2003, 125, 6034-6035. The reaction in this article
follows a Wittig-type process, creating a product that is highly
E-selective. “Total
synthesis of (+)-phorboxazole A, a potent cytostatic agent from the
sponge
Phorbas sp.” Pattenden, G.; González, M.A.;
Little, P.B.; Millan, D.S.; Plowright, A.T.; Tornos, J.A.; Ye, T. Organic
and
Biomolecular Chemistry 2003,
1, 4173-4208. “Wittig-type
olefination catalyzed by PEG-telluride” Huang, Z.-Z.; Ye, S.; Xia, W.; Yu,
Y.-H.; Tang, Y. Journal of
Organic Chemistry 2002, 67, 3096-3103. Papers
that cited “Generation of Phosphoranes” “Reactivity
and selectivity in the Wittig reaction: A computational
study” Robiette, R.; Richardson, J.;
Aggarwal, V.K.; Harvey, J.N. J. Am. Chem.
Soc. 2006, 128,
2394-2409. The authors cited
“Generation
of Phosphoranes” to provide an example for Witteg Olefination. The article studies the
possibilities with
this olefination, and “Generation of Phosphoranes”
serves as an example. “A
practical, efficient, and atom economic alternative to the Wittig and
Horner-Wadsworth-Emmons reactions for the synthesis of
(E)-α,β- unsaturated
esters from aldehydes” List, B.; Doehring, A.; Hechavarria
Fonseca, M.T.; Job, A.; Rios Torres, R. Tetrahedron 2005, 62,
476-482. The authors cited
“Generation
of Phosphoranes” to show a traditional example of Wittig
Olefination and
proceeds to offer an alternative to this traditional method. “On
the origin of high E selectivity in the Wittig reaction of stabilized
ylides:
Importance of dipole-dipole interactions” Robiette, R.; Richardson, J.;
Aggarwal, V.K.; Harvey, J.N. J. Am. Chem.
Soc. 2005, 127,
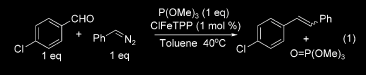
13468-13469. Why "Generation of Phosphoranes?" “Generation
of Phosphoranes Derived from Phosphites. A
New Class of Phosphorus Ylides Leading to High ESelectivity with
Semi-stabilizing
Groups in Wittig Olefinations” The reaction mechanism found in this paper is very similar to the mechanism of the Wittig Olefination found in the transformation of 69 to 70. In this paper, the authors react hydrazones with aldehydes in order to form a carbon-carbon double bond in place of the C=N bond. In this reaction, an SN2 substitution takes place with (MeO)3P being substituted for the two nitrogen molecules, releasing N2. A carbanion is formed on the carbon α to the (MeO)3P, and this carbanion attacks the electrophilic carbon of the aldehyde, and via a cyclic intermediate, it forms primarily the final olefinated trans product. This type of reaction mechanism is important to the understanding of the transformation of 69 to 70, in which an aldehyde is converted to an alkene by basically the same mechanism; the only difference is that 70 is the kinetic product that results from the cis ylide intermediate and therefore forms the E stereoisomer.
|