
Citation Page
Original Source (cited by our article): (10) Dess, D. B.; Martin, J. C. J. Am. Chem. Soc. 1991, 113, 7277-7287.
1. Sorensen, A. M.; Nielsen, K. E.; Vogg, B.; et al Tetrahedron. 2001, 57, 10191-10201.
Synthesis and NMR-studies of dinucleotides with conformationally restricted cyclic phosphotriester linkages
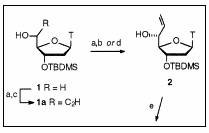
Dess-Martin periodinane oxidation step[1]
The
easily available, protected, thymidine derivative 1 was
oxidized using the Dess�Martin periodinane oxidation and used in a Grignard
reaction, with the application of a commercial solution of vinyl magnesium
bromide. This reaction yielded isomers 2 and 3
in an equimolar ratio, but in a relatively low yield (41%) (33% yield over the
two steps). The product was isolated as a clear oil and as a ![]() 1:1
mixture of epimers. The yield was 620 mg, 41%. Other Grignard reactions that
have been performed on the same aldehyde have reported similar low percent
yields, especially due to a reductive side-reaction resulting in the starting
material. As an alternative to the vinyl Grignard reaction above, the use of an
acetylenic Grignard reagent followed by a reduction reaction using the Lindlar
catalyst was applied. Therefore, the Grignard addition yielded �the epimeric
mixture 1a in 52% yield followed by a reduction in 98% yield to
give the mixture of 2 and 3 in 50% overall
yield from 1� (5.3: Chemical Preparations)
1:1
mixture of epimers. The yield was 620 mg, 41%. Other Grignard reactions that
have been performed on the same aldehyde have reported similar low percent
yields, especially due to a reductive side-reaction resulting in the starting
material. As an alternative to the vinyl Grignard reaction above, the use of an
acetylenic Grignard reagent followed by a reduction reaction using the Lindlar
catalyst was applied. Therefore, the Grignard addition yielded �the epimeric
mixture 1a in 52% yield followed by a reduction in 98% yield to
give the mixture of 2 and 3 in 50% overall
yield from 1� (5.3: Chemical Preparations)

Isomers 2 and 3
This chemistry is seen in our experimental in steps 21 to 22 and 22 to 23.
Reagents and conditions: (a) Dess�Martin periodinane, CH2Cl2; (b) vinylMgBr, THF, 33% (2 steps); (c) ethynylMgBr, THF, 52% (2 steps). The same reagents and conditions were used in our synthesis.
Articles that cite the above article:
1.
McReynolds, M. D.; Dougherty, J. M.; Hanson, P. R.
Chem. Rev. 2004, 104, 2239-2258.
Synthesis of phosphorus and sulfur heterocycles via ring-closing olefin
metathesis
Cited information about past experimentation important to this synthesis:
In 2000, Nielsen and co-workers described the first examples of ring-closing olefin metathesis (RCM) to cyclic phosphates in their efforts to generate conformationally restricted dinucleotides.
2.
Freitag, M.; Thomasen, H.; Christensen, N. K.; et al. Tetrahedron 2004,
60, 3775-3786.
Ring-closing metathesis based synthesis of bicyclic nucleosides locked in
S-type conformations by hydroxyl functionalised 3 ',4'-trans linkages
Introduction:
The biological importance of the conformational equilibrium of nucleosides
between N-type and S-type conformations following the
pseudorotational cycle[1.
and
2.] has motivated the preparation of a significant number of synthetic
nucleoside analogues mimicking these conformational ranges. Thus, nucleosides
with conformationally restricted bi- or tricyclic carbohydrate parts have been
intensively studied as building blocks in nucleic acid analogues, (dinucleotides
with conformationally restricted cyclic phosphotriester linkages) and/or for
potential antiviral agents.
3.
Borsting, P.; Freitag, M.; Nielsen, P.
Tetrahedron 2004, 60, 10955-10966.
Dinucleotides containing two allyl groups by combinations of allyl phosphotriesters, 5-allyl-, 2 '-O-allyl-
and 2 '-arabino-O-allyl uridine derivatives as substrates for ring-closing metathesis
Introduction:
Neilson was a co-author of this paper, and cited his own paper as a reference and as background material for this paper. He describes how they have focused on the application of RCM in nucleic acid chemistry, and as a result, conformationally restricted bi- and tricyclic nucleoside monomers as well as di- and trinucleotides with large cyclic structures have been achieved.
Other References:
Dess, D. B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155-4156.
Dess, D. B.; Martin, J. C. J. Am. Chem. Soc. 1991, 113, 7277-7387.
�Dess-Martin Periodinane� (http://en.wikipedia.org/wiki/Dess-Martin_oxidation)
Accessed 3/19/06.
�Dess-Martin Oxidation�
(http://www.organic-chemistry.org/namedreactions/dess-martin-oxidation.shtm )
Accessed 3/19/06.
[1] Note: the oxidized intermediate is not pictured in the reaction sequence because there is a sequence of 2 steps for the DMP reaction; therefore, the actual oxidized form may have been the intermediate, thus not pictured.