 |
       |
Experimental |
| Sulfonamide 30 |
A solution of 3-butyn-1-ol (6.9 mL, 88.0 mmol), triphenylphosphine (38.5 g, 146 mmol), N-tert-butoxycarbonyl-p-toluenesulfonamide (19.9 g, 73.3 mmol) were placed in 400 mL of THF and cooled in an ice bath under a blanket of nitrogen. Diethyl azodiformate (20.8 mL, 131.9 mmol) was added dropwise to the solution. The ice bath was removed and the solution was stirred for 24 hours. The mixture was concentrated under reduced pressure, absorbed onto silica gel and purified by flash chromatography (1:7 EtOAc-Hexanes, and then 1:5 EtOAc-Hexanes) to yield sulfonamide 30 (20.9 g, 88%) as a colorless oil. 1H-NMR (200 MHz, CDCl3) d 7.8 (d, 2H), 7.3 (d, 2H), 4.0 (t, 2H), 2.7 (s, 1H), 2.5 (t, 2H), 2.0 (s, 3H), 1.4 (s, 9H). |
1Becker, M.H.; Chua, P.; Downham, R.; Douglas, C.J.; Garg, N.K.; Hiebert, S.; Jaroch, S.; Matsuoka,
R.T.; Middleton, J.A.; Ng, F.W.; Overman, L.E. J. Am. Chem. Soc. 2007, 129, 11987-12002.
2http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed Feb 19 2008) |
|
 |
| Glossary of terms: |
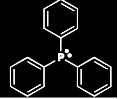
| 1 Triphenylphosphine1
Molecular Formula: C18H15P
Molar Mass: 262.3 g/mol
Apperance: White Solid (crystal)
Density: 1.1 g/cm3
Melting Point: 80 °C
Boiling Point: 377 °C |
 |
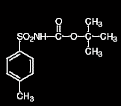
| N-tert-butoxycarbonyl-p-toluenesulfonamide
Molecular Formula: C12H17NO4S
Molar Mass: 271.3 g/mol |
 |
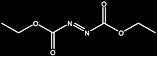
| Diethyl azodiformate
Molecular Formula: C6H10N2O4
Molar Mass: 174.2 g/mol |
 |
| Flash Chromatography
A version of column chromatography in which air pressure is applied to the top of the column to speed the flow of solvent through the silica bed. |
 |
|
|
 |
1Triphenylphosphine. (2008, February 22). In Wikipedia, The Free Encyclopedia. Retrieved, April 2, 2008, from http://en.wikipedia.org/w/index.php?title=Triphenylphosphine&oldid=193173749 |
Designed by Nikola Collins, James DeRosier and Justin
Zehtabzadeh - April 2008
|
|




