 |
       |
References |
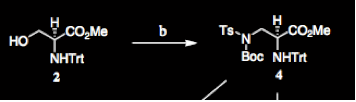
One of the references that our paper cited1 dealt with the Mitsunobu reaction, which adds an (Boc)TsN group to a molecule by utilizing Ph3P. Our article cited this specific reference because our reaction can also be completed using this mechanism (the earlier mechanism involved a tosylation and nucleophilic substitution). |
 |
| 1Henry, J.R.; Marcin, L.R.; McIntosh, M.C.; Scola, P.M.; Harris, G.D., Jr.; Weinreb, S.M. Tetrahedron Lett. 1989, 30, 5709-5712. |
 |
A) The first paper that also cited this reference and used the same material:
Bohno, M.; Chida, N.; Imase, H.; Oishi, T.; Sugie, K.; Yusof, Y.B. Tetrahedron. 2007, 63, 6977-6989. |
| They also utilized the Mitsunobu reaction: |
|
 |
B) The second paper that also cited this reference and used the same material:
Ando, K.; Chiba, S.; Fukamizu, K.; Miyauchi, H.; Narasaka, K. Tetrahedron. 2007, 63, 5940-5953. |
| The relevant chemistry is again the Mitsunobu conditions, which replace the hydroxyl group. |
|
|
 |
C) The third paper that cited this source was:
Kelleher, F.; Proinsias, K. Tetrahedron Lett. 2007, 48, 4879-4882 |
Again, the relevant chemistry in this article is the Mitsunobu conditions. The article explains that they “decided to examine the Mitsunobu reaction of 1-serine derivatives with nitrogen-based nucleophiles, other than azide, for their synthesis. Our first choice was sulfonamide based nucleophiles (Ts or Ns) because they have appropriate pKa values. In 1989 Weinreb introduced Ts-NH-Boc in the Mitsunobu reaction for the conversion of alcohols into N-Boc p-toluenesulfonamides in excellent yields.” |
 |
|
| |
|
Designed by Nikola Collins, James DeRosier and Justin
Zehtabzadeh - April 2008
|
|