   


|
Epoxidation of Alkenes and Heterocyclic C=C bonds using Aromatic Peroxyacids...duh
Epoxides, also known as oxiranes, are three-member cyclic ethers with an approximate bond angle of 60°. Because of the large amounts of angle strain in this small ring, epoxides undergo acid and base-catalyzed C-O bond cleavage more easily than larger ring ethers. The ring strain also makes epoxides highly reactive toward nucleophiles and they are known to undergo SN2 type of ring-opening reactions. The exceptional reactivity of epoxides makes them an excellent intermediate in industrial syntheses of various products, such as perfumery chemicals, pharmaceuticals, and agrochemicals.
Due to epoxide’s wide variety of synthetic application, its preparation becomes a technically important process. Epoxides are preferably formed by the reaction of an alkene with an oxidizing agent in the presence of a catalyst. Various oxidizing agents such as commercial bleach, organic hydroperoxides, organic per acids, iodosyl arines, oxones, molecular oxygen (in the form of pure oxygen or atmospheric oxygen) and hydrogen peroxide have been used to prepare a variety of alkene epoxides.
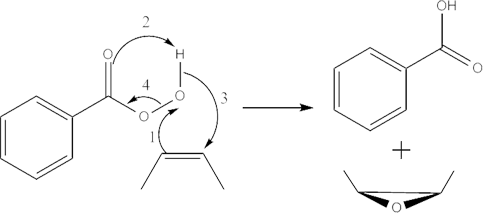
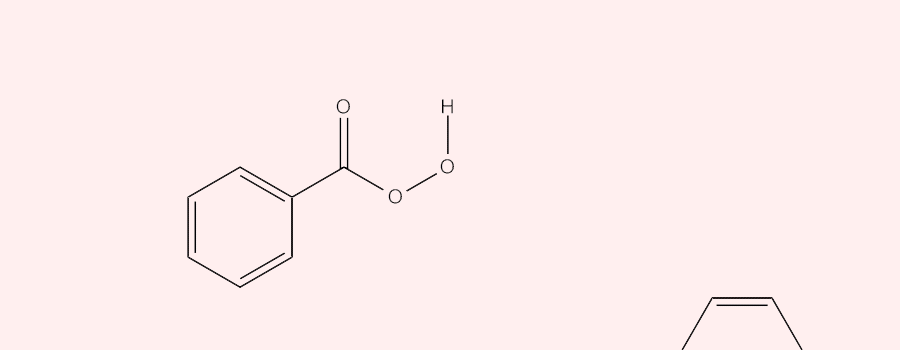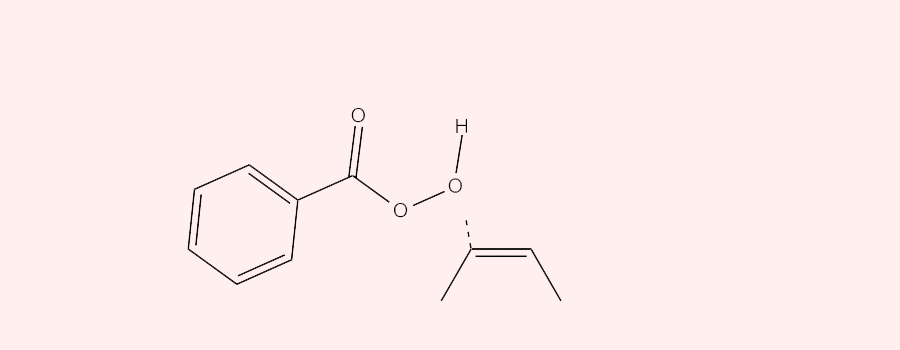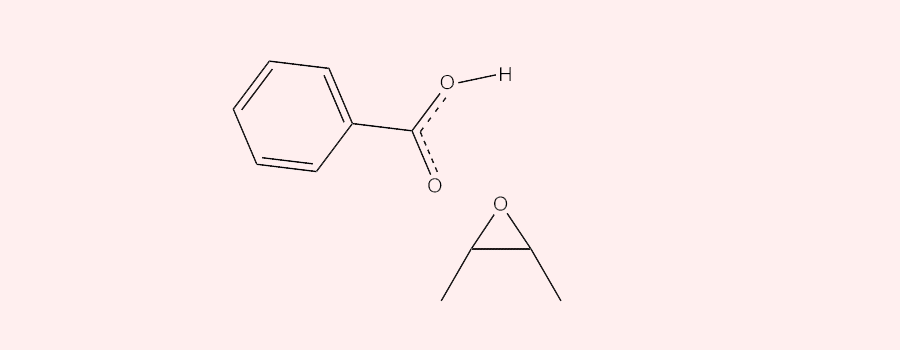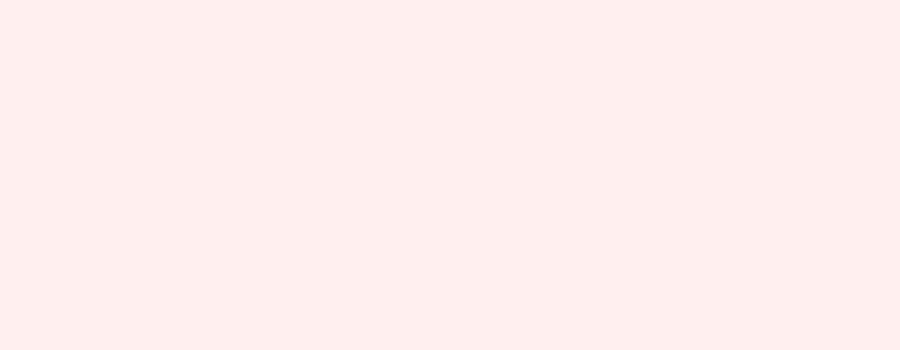
The epoxidation of alkenes and heterocyclic carbon double bonds with peroxybenzoic acid (ArCO3H) involves a concerted oxidation reaction in which an oxygen atom is transferred from the peroxybenzoic acid to the double bond. The reaction starts with the peroxybenzoic acid behaving as an electrophile and selectively attacking the double bonds having different degrees of substitution. The more substituted (the higher electron density) the double bond, the more readily it is attacked by the nucleophilic oxygen, the one directly attached to the hydrogen, in the peroxybenzoic acid. Meanwhile, during the attack, one of the carbons in the double bond attacks the lone pair in the Oxygen from the O-H bond in the peroxybenzoic acid and the proton is released. The oxygen gets transferred as the sigma O-O bond in peroxybenzoic acid cleaves off to facilitate the reaching out of C=O bond to hold onto the released proton.
Return to SSG 3 Main Page
|








