Into a stirred solution of 39 (6.25 g, 16.9 mmol) in toluene (34 ml) was bubbled ozone at 0oC. After TLC analysis showed a complete consumption of olefin 39, triphenylphosphine (4.88 g, 18.6 mmol) was added and the solution was stirred at room temperature for 30 min. To the resulting solution was added 50% aqueous trifluoroacetic acid (10 ml) at room temperature. After stirring at 800C for 2 h, the solvent was removed in vacuo. The residue was dissolved in ethyl acetate and neutralized with aqueous sodium bicarbonate, and the solution was extracted with ethyl acetate. The combined organic solution was washed with brine, dried over sodium sulfate, and filtered. The filtrate was concentrated in vacuo and the residue was purified with flash column chromatography (50% ethyl acetate in n-hexane) to afford 40 (5.51 g, 85%) as a yellow oil. IR (film) 1669, 1550, 1539, 1352, 1164 cm-1; 1H NMR (400 MHz, CDCl3)δ 8.49(dd,J=8.7Hz,2.3Hz,1H),8.44(d,J=2.3Hz,1H),8.21(d,J=8.7 Hz, 1 H), 6.86 (t, J = 4.2 Hz, 1 H), 3.34 (t, J = 6.9 Hz, 2 H), 2.96 (s, 3 H), 2.37-2.49 (m, 6 H), 2.00 (m, 2 H); 13C NMR (100 MHz, CDCl3) δ 199.2, 149.6, 149.6, 148.5, 138.2, 135.5, 132.7, 126.0, 119.6, 49.7, 38.2, 34.6, 28.7, 26.1, 22.9; HRMS (ESI) calcd for C15H17N3O7SNa (M+Na)+: 406.0685, found 406.0672.

![N-methyl-N-[2-(6-oxocyclohex-1-enyl)ethyl]-2,4-dinitrobenzenesulfonamide (40)](Experimental_files/shapeimage_2.png)









Bubbled Ozone1:
Ozone can be bubbled into solution with a bubble diffuser. The deeper in solution the bubble diffuser is placed, the more effectively the ozone is dissolved into the solution without escaping.
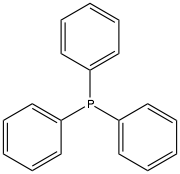
Triphenylphosphine
Triphenylphosphine is often used in the synthesis of organic and organometallic compounds. The use of triphenylphosphine in ozonolysis produces aldehydes or ketones while the use of sodium borohydride would produce alcohols.
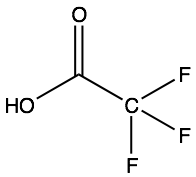
Trifluoroacetic Acid4
Trifluoroacetic acid is a very strong carboxylic acid due to its electronegative trifluoromethyl group. It has a pKa around -0.25. This triple-fluorinated version of acetic acid is nearly a hundred thousand times stronger than regular acetic acid.
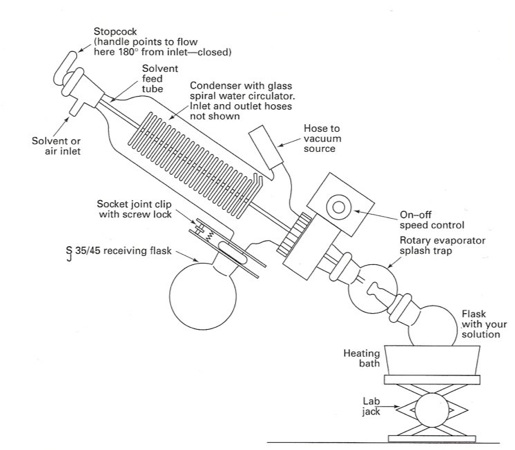
In vacuo6
When your product is dissolved in a solvent, you can easily evaporate the solvent off using a rotary evaporator, or a rotovap. The rotovap will rotate your flask under a vacuum in a heating bath, which increases the surface area of the solvent to speed up the evaporation process.

































Flash Column Chromatography
This procedure uses an ordinary wet-column chromatography setup, but is put under pressure in order to quickly purify organic compounds.
Welcome to the carbo-jungle. Check out this EXPERIMENTAL PROCEDURE.







