Molecule 29

Tan! A little bit of UV and NMR all in one!
 |
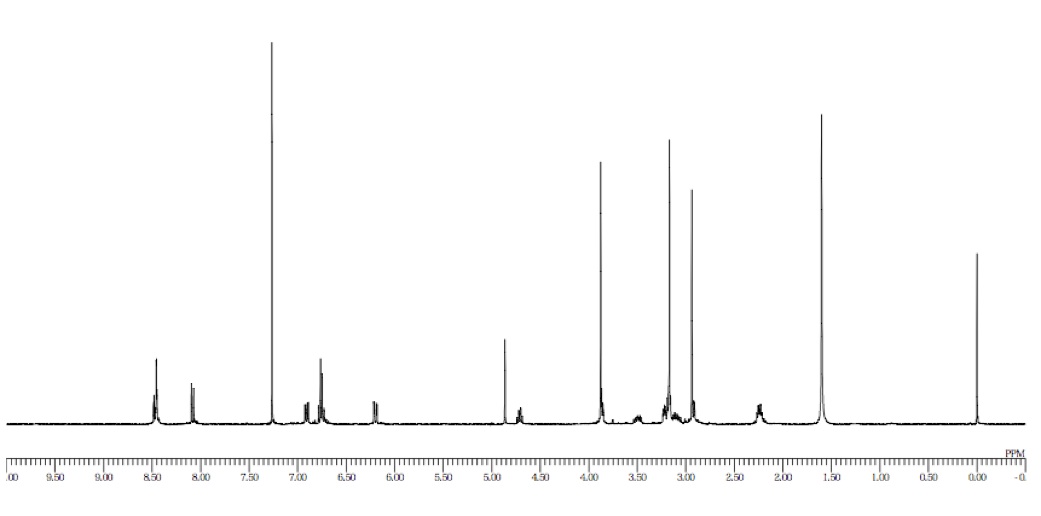
a: The first peak at 8.49 ppm correlates to the hydrogen atom that is between two nitro groups on the aromatic ring, causing it to have a high downfield shift.
b: The second peak at 8.45 ppm correlates to one hydrogen atom on the aromatic ring that is shifted downfield and couples with two hydrogens, producing a doublet of doublets.
c: The third peak at 8.21 ppm correlates to one hydrogen on the aromatic ring that is farther from the two nitro groups, giving it a lesser downfield shift.
d: The fourth peak at 5.35 ppm corresponds to a non-aromatic hydrogen that is bonded to an sp2 carbon. It has an integration of 1H and has a triplet splitting pattern because it is coupled with the two diastereotopic vicinal neighbors.
e: The fifth peak at 3.25 ppm corresponds with two hydrogen atoms that are de-shielded by the nearby nitrogen atom. They are coupled with two vicinal neighbors, producing a doublet.
f: The sixth peak at 2.94 ppm corresponds to 3 hydrogen atoms that are de-shielded by an electronegative nitrogen atom. It shows up as a singlet due to absence of vicinal or geminal neighbors.
g: The seventh peak at 2.30 ppm corresponds to two hydrogen atoms that couple to form a multiplet. This is due to coupling with Hd, and the two diastereotopic Hj atoms.
h: The eighth peak at 2.21 ppm corresponds with two hydrogen atoms of the cyclopentene that couple with Hj to form a triplet
i: The ninth peak at 2.09 ppm corresponds to the two hydrogen atoms of the carbon chain closest to the cyclopentene.
j: The tenth peak at 1.84 ppm corresponds to the two hydrogen atoms of the cyclopentene that are farthest from the double bond.
k: The eleventh peak at 1.76 ppm corresponds to the two hydrogen atoms of the carbon chain that has two sets of neighbors, producing a doublet of doublets.
Molecule 39
 |
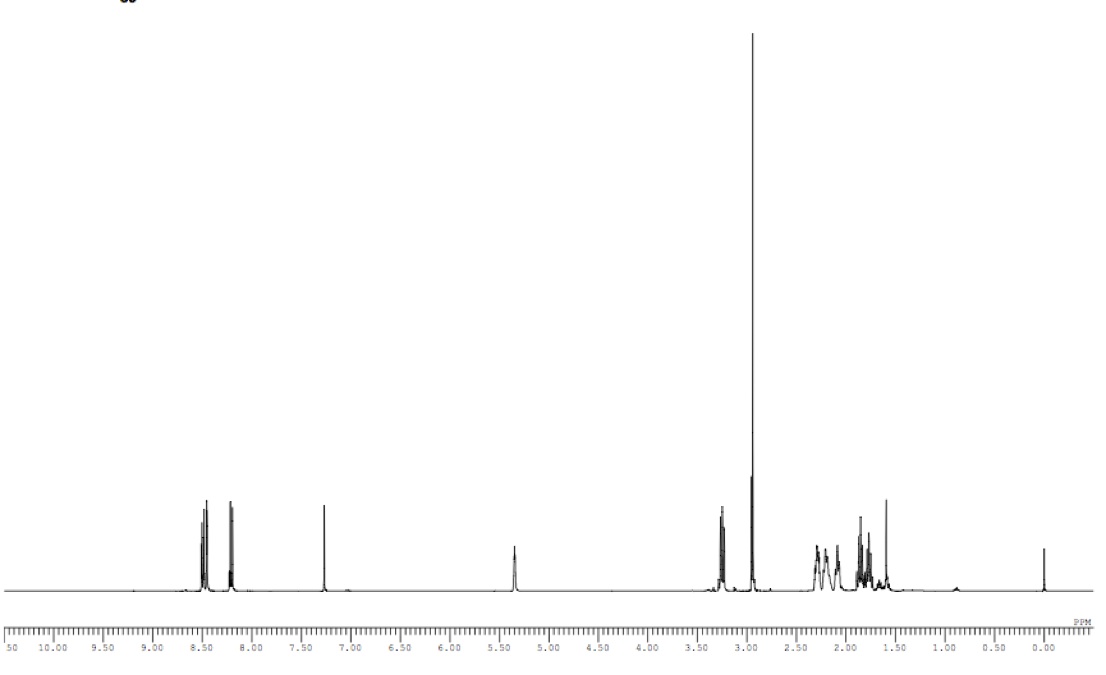
a: The first peak at 8.48 ppm correlates to the hydrogen atom that is between two nitro groups on the aromatic ring, causing it to have a high downfield shift.
b: The second peak at 8.46 ppm correlates to one hydrogen atom on the aromatic ring that is shifted downfield and couples with two hydrogens, producing a doublet of doublets.
c: The third peak at 8.08 ppm correlates to one hydrogen on the aromatic ring that is farther from the two nitro groups, giving it a lesser downfield shift.
d: The fourth peak at 6.91 ppm correlates to one hydrogen atom bonded to an sp2 carbon atom that is also de-shielded by a ketone. It is coupled with a cis Hg with a coupling constant of 10.5 Hz, as well as an sp3 vicinal neighbor with a coupling constant of 4.0 Hz.
e: The fifth peak at 6.77 ppm correlates to an aromatic hydrogen that is coupled with Hf with a coupling constant of 8.2 Hz.
f: The sixth peak at 6.74 ppm correlates to an aromatic hydrogen that is coupled with He with a coupling constant of 8.2 Hz.
g: The seventh peak at 6.20 ppm correlates to a hydrogen atom that is bonded to an sp2 carbon atom that is coupled with Hd with a coupling constant of 10.5 Hz because they are cis on the alkene.
h: The eighth peak at 4.86 ppm correlates to a single hydrogen atom that is de-shielded by two nearby oxygen atoms, and produces a singlet due to a lack of neighbors.
i: The ninth peak at 4.71 ppm correlates to a single hydrogen that is de-shielded by the OMs group and produces a doublet of doublets.
j: The tenth peak at 3.88 ppm correlates to three homotopic hydrogen atoms that are de-shielded by an oxygen atom.
k: The eleventh peak at 3.50 ppm correlates to a single hydrogen atom that produces a complex multiplet splitting pattern.
l: The twelfth peak at 3.22 ppm correlates to a single hydrogen atom that is coupled with Hi with a coupling constant of 6.2 Hz, as well as Hd with a coupling constant of 4.0 Hz, producing a doublet of doublets.
m: The thirteenth peak at 3.16 ppm correlates to three homotopic hydrogen atoms that slightly de-shielded by electronegative sulfur and oxygen atoms.
n: The fourteenth peak at 3.16 ppm correlates to a single hydrogen atom that is diastereotopic to Hk, and is shifted downfield by the nearby aromatic ring and electronegative oxygen atom.
o: The fifteenth peak at 3.10 ppm correlates to a single hydrogen that is slightly de-shielded by a nearby nitrogen atom, and produces a complex multiplet splitting pattern.
p: The sixteenth peak at 2.94 ppm correlates to three homotopic hydrogen atoms that are slightly de-shielded by a nearby nitrogen atom.
q: The seventeenth peak at 2.91 ppm correlates to a single hydrogen that produces a complex multiplet splitting pattern and is de-shielded by a nearby nitrogen atom.
r: The eighteenth peak at 2.23 ppm correlates to two hydrogen atoms that produce a complex multiplet splitting pattern.