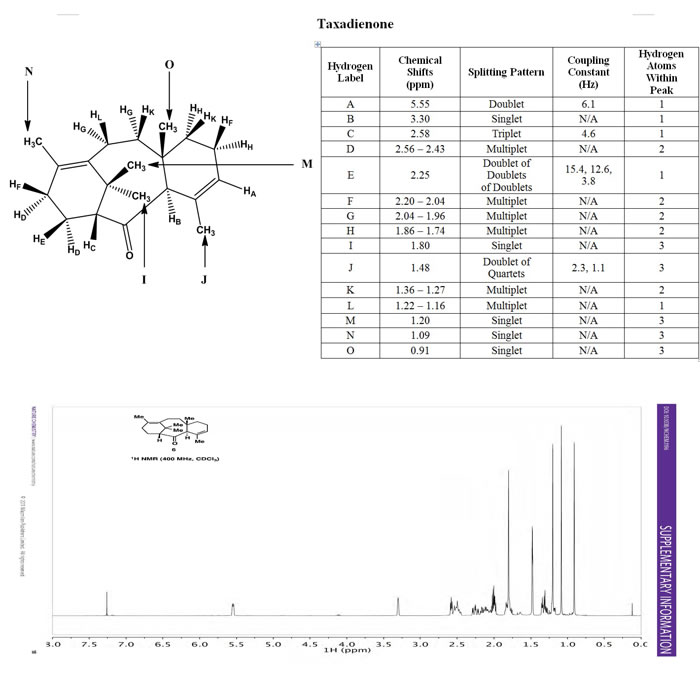
A: Is the most downfield since it is directly attached to a carbon-carbon double bond. The doublet nature of hydrogen A is quite perplexing. I believe that hydrogen A is actually a triplet since it has two three bond neighbors, it is just that the coupling constants of hydrogen F and hydrogen G are so similar that it gives the appearance of a doublet for hydrogen A.
B: It is the next most downfield since it is closer to the oxygen, which is pointing away from hydrogen C, but is pointing towards hydrogen B. Hydrogen B also has no three bond neighbors, and therefore is a singlet. It is also attached to a tertiary, sp3 carbon.
C: Is the next most downfield since it is close to the oxygen, even though both atoms are pointing in opposite directions. It is also attached to a tertiary, sp3 carbon. It is a triplet because hydrogen atoms E and D are three bond neighbors to it.
D: These are two hydrogen atoms that point into the cavity of the cyclohexene towards the carbonyl group. This makes them more downfield than the hydrogen atoms point out from the cavity of cyclohexene. These hydrogen atoms are also closer to the carbonyl group than the hydrogen atoms on the other cyclohexene.
E: This is the next most downfield due to its proximity to the carbonyl group. It is also a doublet of doublets of doublets, meaning the peak gets split thrice by chemically different three-bond hydrogen neighbors. This is the case for hydrogen E, since it is three-bond neighbors to hydrogen atoms C, D, and F.
F: This is a multiplet of two hydrogen atoms. Hydrogen atoms F are both in similar chemical environments due to an equal bond distance from an alkenyl group , causing them to have similar chemical shifts. These hydrogen atoms also have many three-bond hydrogen neighbors and will form many overlapping peaks, which constitutes a multiplet.
G: Hydrogen G is also a multiplet for the same reasons as stated above. Hydrogen G is also pointing into the cavity of the cyclohexene, towards the oxygen and is relatively close to the carbonyl groups and an alkenyl group.
H: Hydrogen H is similar to hydrogen G except hydrogen H is more upfield, this is because it is farther away from the carbonyl group.
I: Hydrogen atoms I represent three hydrogen atoms and must be a methyl group. It is also the most downfield methyl group. Therefore hydrogen atoms I are the methyl group pointing into the cavity of the cyclohexene closest to the carbonyl group.
J: Hydrogen atoms J are the next most downfield methyl group. These must be hydrogen atoms in the methyl group closest to the carbonyl that is attached to an sp2 carbon. Since all the hydrogen atoms in the methyl groups do not have three-bond hydrogen neighbors, I believe hydrogen atoms J are long distance coupling with hydrogen atoms within the cyclohexene ring to form a doublet of quartets.
K: These hydrogen atoms are both on wedges and one bond away from a quaternary carbon. These hydrogen atoms are both three bonds away from an sp2 carbon as well. Due to these similarities, these hydrogen atoms should have similar chemical shifts. These hydrogen atoms also have many three bond neighbors to form many overlapping peaks, a multiplet.
L: This hydrogen is the least downfield single hydrogen because it is on a wedge attached to an sp2 carbon, locking the carbon attached to hydrogen L in-plane with the sp2 carbon, across from the carbonyl group. The carbonyl group is pointing into the plane and outwards from hydrogen L. This makes hydrogen L farther away from the carbonyl that hydrogen atoms K and G, which are attached to a carbon that is pointing somewhat into the plane, similar to the carbonyl. Due to this distance, hydrogen L is the most upfield single hydrogen.
M: These hydrogen atoms are part of a methyl group and are closest to the carbonyl, but pointing away from it.
N: These hydrogen atoms are connected to a primary carbon that is bonded directly to an sp2 carbon, making it more downfield than hydrogen atoms O.
O: These hydrogen atoms are connected to a primary carbon that is not directly attached to a more electronegative carbon. Therefore hydrogen atoms O should be the least downfield. |