Original Article
Mendoza, A.; Ishihara, Y.; Baran, P. S. Nature Chem. 2012, 4, 21-25.
Articles that include Negishi Coupling
Coleridge, B. M.; Bello, C. S.; Ellenberger, D. H.; Leitner, A. Tetrahedron Lett. 2010, 51, 357-359.
Kaae, B. H.; Krogsgaard-Larsen, P.; Johansen, T. N.; J. Org. Chem. 2004, 69, 1401-1404.
Zhang, T.; Gao, X.; Wood, H. B.; Tetrahedron Lett. 2011, 52, 311-313.
Summary for Leitner et al.:
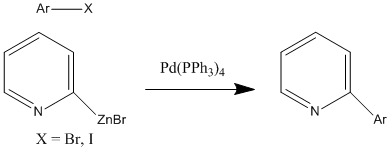
Reaction Scheme for Leitner et al. This paper seeks to cross-couple 2-pyridylzinc bromide with various aryl halides via Negishi coupling. What is unique about this study is that it is the first study of 2-pyridylzinc bromide and its value as a reagent in Negishi coupling. Specifically, this study investigates the reactivity of 2-pyridylzinc bromide and aryl bromides and iodides. It concluded that when these reagents are coupled, it can be done in a step and with high yield. Additionally, this Negishi coupling reaction does not require specific reaction conditions or any additives for optimal results, meaning that this reaction is effective. Thus, the study has found that Negishi coupling can be a highly effective method to form pyridine-aryl adducts.
Articles Citing Leitner et al.
1. Ren, W.; Li, J.; Zou, D.; Wu Y.; Wu, Y. Tetrahedron 2012, 68, 1351–1358.

Relevant Chemistry: Used Negishi coupling to create substituted heterobiaryls.
Jonathan - This was mentioned because they needed a way to create substituted heterobiaryls.
Justin - The authors mentioned the Leitner article because they used the Leitner method, Negishi coupling, to obtain pyridine and benzene adducts.
Collin - The authors wanted to mention the various ways for Negishi coupling to occur with transition-metal-catalyses.
2. Blakemore, D. C.; Marples, L. A. Tetrahedron Lett. 2011, 52, 4192-4195.

Relevant Chemistry: Used Negishi coupling to create dipyridyl structures.
Jonathan- This was mentioned because the products created in the Leitner et al. article could not be isolated in situ.
Justin - The authors mentioned the Leitner article because the products that the Leitner group created could not be isolated in a desired way.
Collin - Leitner et al. was cited because their synthesis of compounds with pyridine and aryl groups, even though the method used was undesirable, were used as a launching point for the Marples study.
3. Luzung, M. R.; Patel, J. S.; Yin, J. J. J. Org. Chem. 2010, 75, 8330-8332.
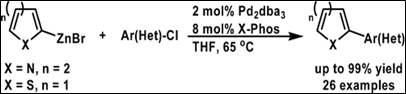
Relevant Chemistry: Used Negishi coupling to bypass Suzuki coupling to make arylic-heterocyclic compounds.
Jonathan - They cited the Leitner et al. because they wanted an alternative method to Suzuki reaction by using Negishi coupling.
Justin - The authors cited the Leitner article because they used the Leitner method to create pyridine-benzene adducts in a way that was deemed efficient.
Collin - Leitner et al. was cited because their Negishi coupling method was used as an example of bypassing the use of Suzuki coupling for making arylic compounds attached to pyridine. |




