

Main Reference: Bian, M.; Wang, Z.; Xiong, X.; Sun, Y.; Matera, C.; Nicolaou, K. C.; Li, A. J. Am. Chem. Soc. 2012, 134, 8078-8081.
Trans-Acetalization Reference: Zhao, M. M.; McNamara, J. M.; Ho, G-j.; Emerson, K. M.; Song, Z. J.; Tschaen, D. M.; Brands, K. M. J.; Dolling, U-h.; Grabowski, E. J. J.; Reider, P. J. J. Org. Chem. 2002, 67, 6743–6747.
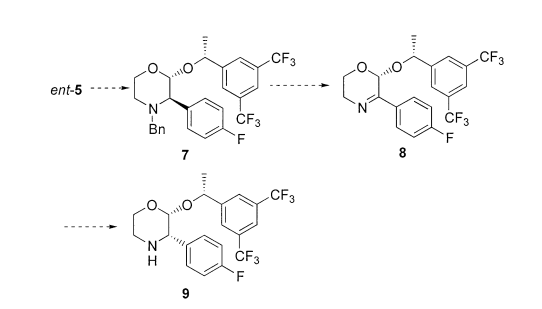
The researchers of this fabulous publication in the Journal of Organic Chemistry discovered an efficient process of producing molecule 9 in high yield. They realized that a Lewis Acid catalyst was essential to this chemical synthesis. The intelligent scientists connivingly conducted a transformational trans-acetalization synthesis reaction of molecule 7 to produce intermediate imine 8 which then was modified to produce a meaningful molecule, which is labeled as compound 9 in the above reaction scheme. A Lewis Acid catalyst is essential to this synthesis reaction in order to produce the desired product with the correct stereochemistry. In the reaction scheme that is shown above, a Lewis Acid catalyzes the tantalizing transformation of molecule 7. This prominent process produces imine 8. Then, the chivalrous chiral center is inverted in imine 8 through a horrendous hydrogenation process. This yields the fantastic final product, product 9.
Addtional References:
Hosokawa, T.; Nakajima, F.; Iwasa, S.; Murahashi, S-I. Chem. Lett. 1990, 19, 1387-1390.
Inoue, M.; Urabe, D. Tetrahedron 2009, 65, 6271-6289.