1
2
3
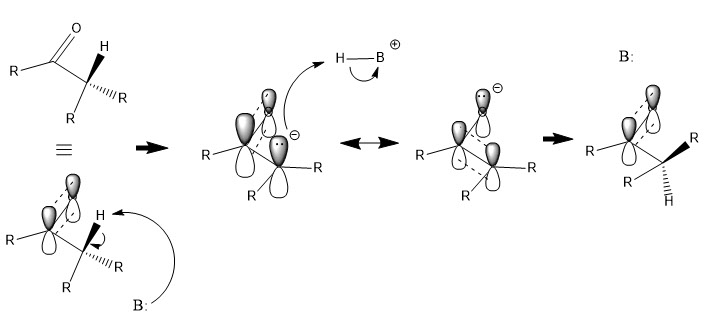
EPIMERIZATION
Epimerization is the inversion of a stereocenter on a molecule. In this experiment, epimerization is achieved through the deprotonation of the carbon stereocenter. With a carbonyl group bonded to the resulting carbanion, resonance will occur and the carbanion will have planar sp2 hydrid orbitals. Since the carbon has low electronegativity, the non-bonding electrons on the carbon will represent the HOMO of the molecule, and so the negatively charged carbon will be protonated by an acidic species. Protonation of the carbanion will occur at the side resulting in the most stable product.
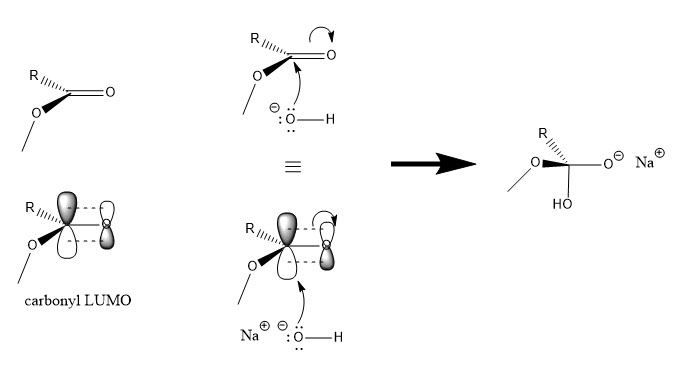
TETRAHEDRAL INTERMEDIATES IN CARBONYL ADDITION
The LUMO of the carbonyl group has a large electron probability density near the less electronegative carbon atom, resulting in the observed electrophilicity of the carbon atom in the carbonyl group. A nucleophilic species with high-energy non-bonding electron HOMO can transfer electrons from its HOMO to the LUMO of the sigma bond. The resulting product is a tetrahedral intermediate in which a new sigma bond forms between the nucleophile and the electrophilic carbon on the carbonyl group, and the pi bond on the carbonyl group breaks to form a carbon-oxygen sigma bond along with an oxygen anion.
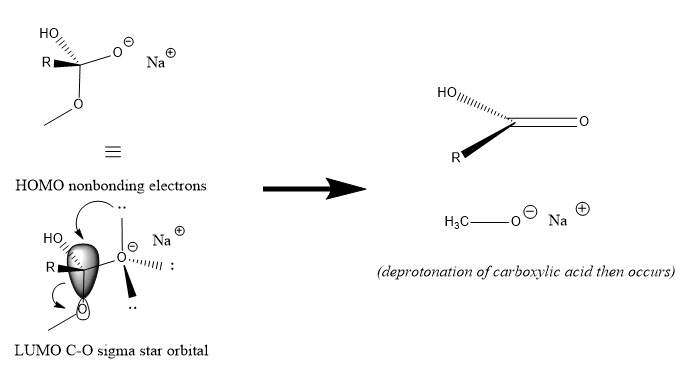
LONE-PAIR ASSISTED IONIZATION
Lone-pair assisted ionization involves the transfer of the non-bonding electrons from high-energy (usually anionic) HOMO to the LUMO of a sigma bond. In this case, the HOMO of the molecule are the anionic oxygen non-bonding electron pairs. A pair of non-bonding electrons can add to the antibonding orbital of the carbon-oxygen bond of the leaving group. A new pi-bond forms in the reaction from the non-bonding electrons, and the sigma bond, resulting in the liberation of the leaving group.
University of Michigan Chem 215/216 HH Winter 2014. Nicholas Carducci's Structured Study Group. HTML Project of Callie Chappell, James Lawniczak, Aiman Faruqi, and Ryan Gentil
