
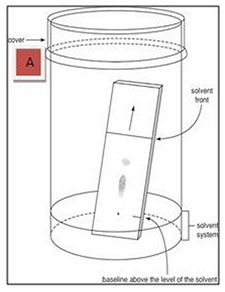
Thin layer chromatography is used primarily in the identification of compounds. Compounds move up the TLC plate based on polarity. Since the silica gel on the plate is highly polar, polar compounds adhere more strongly to the plate and move more slowly with the solvent up the plate (smaller retention factor, Rf). Conversely, nonpolar compounds have weaker interactions with the polar plate and stronger interactions with the comparatively more nonpolar solvent, moving farther up the plate (larger retention factor, Rf). Here, the reaction mixture was plated on 0.25 mm E. Merck silica gel plates (60F-254) and was visualized using an UV light. The solvent used was an acidic mixture of anisaldehyde, phosphomolybdic acid, or ceric ammonium molybdate, or basic aqueous potassium permangante (KMnO4), and heat.
Zubrick, J. W. The Organic Chem Lab Survival Manual: A Student’s Guide to Techniques, 9th ed.; Massachusetts, 2014; pp 223-233.
University of Michigan Chem 215/216 HH Winter 2014. Nicholas Carducci's Structured Study Group. HTML Project of Callie Chappell, James Lawniczak, Aiman Faruqi, and Ryan Gentil