Now that I have found my voice and gained my land legs with the help of some handy-dandy roidage, I'd like to thank all the people and resources that helped me defeat the evil witch Ursula.

Firstly I'd like to thank Sebastian for showing me the main article that is one of the references of our SSG article: Isayama, S.; Mukaiyama, T. Chem. Lett. 1989, 18, 1071-1074.
This paper is related to the reaction of 34 to 24 as it is the original paper describing and proposing the Mukaiyama Hydration reaction of the first step in our reaction scheme. This article suggests a theoretical mechanism that could explain the cobalt-induced hydration of molecule 34 to 24 in our reaction.
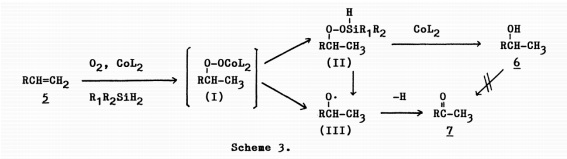
As seen by the proposed mechanism from this article, the molcule of Oxygen gas bonds with a cobalt molecule to form a new comlex that bonds with the C=C, which forms a radical. This radical then undergoes subsequent reactions involving a silicon-based molecule to form the final OH in the desired product. These conditions are extremely similar to those of our reaction, and the starting material and product of the first step of our reaction scheme is analogous to those of this experiment. These would suggest the same mechanistic pathway as the Chemistry Letters article.
I would also like to thank other friends of mine for finding supporting articles that cite the main article provided by Sebastien.

To one of my closest friends, Flounder, for this article: Zahel, M.; Metz, O. Beilstein J. Org. Chem. 2013, 9, 2028-2032. This article was picked because of reaction processes it employed. The chemists behind this experiment used the Mukaiyama Hydration as a proposed step in their synthesis, and cited Sebastien's main paper in order to give it credit and precedence. We essentially picked this article due to this relevant chemistry where they add an OH and an H across a double bond with this particular Hydration.

To my darling lover, Prince Eric, for this article: Farcet, J.-B.; Himmelbauer, M.; Mulzer. J. Eur. J. Org. Chem. 2013, 52, 4379-4398. This article includes an example of a Mukaiyama Hydration used in the same way our paper does. The OH is selectively added pointing downwards to one side of a double bond using the exact same reagents as well. The subsequent steps are slightly different but the point still holds, as can be seen from the excerpt included from the paper.
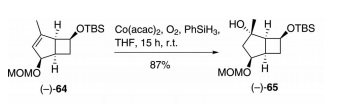

To my dear father, King Triton, for the last article: Shigehisa, H.; Suwa, Y.; Furiya, N.; Nakaya, Y.; Fukushima, M.; Ichihashi, Y.; Hiraya, K. Angew. Chem. Int. Ed. 2013, 52, 3646-3649. This article has very similar, but slightly different reaction conditions and has analogous starting material products, which was one reason why we chose it. However, these chemists proposed a different mechanism to achieve the same thing, even though the cite the original Mukaiyama Hydration article as a reference because of the relevant chemistry contained in it.
And lastly, I would like to give myself for finding the original SSG3 article: Shi, J.; Manolikakes, G.; Yeh, C.-H.; Guerrero, C.A.; Shenvi, R.A.; Shigehisa, H.; Baran, P.S. J. Am. Chem. Soc. 2011, 133, 8014-8027.
Works Cited
Farcet, J.-B.; Himmelbauer, M.; Mulzer. J. Eur. J. Org. Chem. 2013, 4379-4398.
Shenvi, R.A.; Guerrero, C.A.; Shi, J.; Li, C.-C.; Baran, P.S. J. Am. Chem. Soc. 2008. 130, 7241–7243
Shi, J.; Manolikakes, G.; Yeh, C-H.; Guerrero, C.A.; Shenvi, R.A.; Shigehisa, H.; Baran, P.S. J. Am. Chem. Soc. 2011, 133, 8014-8027.
Shigehisa, H.; Suwa, Y. Furiya, N. Nakaya, Y. Fukushima, M.; Ichihashi, Y.; Hiraya, K. Angew. Chem. Int. Ed. 2013, 52, 3646-3649.
Zahel, M.; Metz, O. Beilstein J. Org. Chem. 2013, 9, 2028-2032.






