| SSG HOME |
 |
| MAIN PAGE | |
| EXPERIMENTAL | |
| MECHANISM | |
| H-NMR CORRELATION | |
| LEADING QUESTIONS | |
| REFERENCES | |
| ABOUT US | |
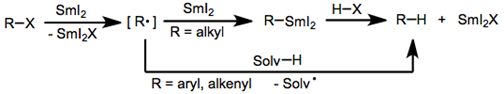
Samarium (II) iodide (SmI2), also known as Kagan’s reagent, is a mild one-electron reducing agent that is often used in the reduction of various classes of organic molecules. This reagent is used to reduce a wide variety of molecules, but more commonly used in the reduction of organic halides and α-functionalized carbonyl compounds. Reactions with SmI2 occur through electron transfer with the reactant, followed by proton transfer from a protic solvent. This causes mechanisms containing SmI2 to contain radical intermediates as well. Reduction of an Organic Halide using SmI2
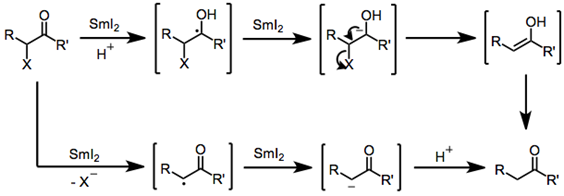
Reduction of a α-Functionalized Carbonyl
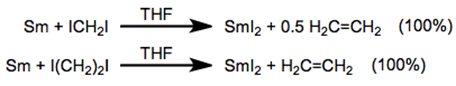
In the preparation of SmI2, samarium metal reacts with either diiodomethane or diiodoethane to produce SmI2. However, diiodomethane is preferred because it is a liquid. Pure SmI2 is air sensitive but in solution it can be manipulated without special precautions because it reacts very slowly with water and alcohol.
|
|