
A disastrous paintball game has left the school a shell of its former self.
Students dart from classroom to classroom in a vision that looks like a scene from an apocalypse
Amidst the ruins of Greendale, one study group must fight...to study another day

DMDO acts on the carbon-carbon
double bond of the molecule to create an epoxide.
This reaction is done in acetone (57%) and DMDO (41%) over a range of
-78 to 23 degrees Celsius. Acetone is formed as a product
of the reaction and adds to the solution.
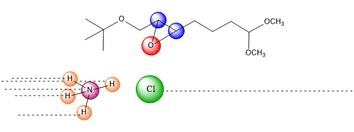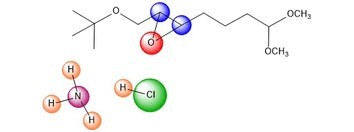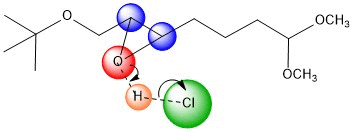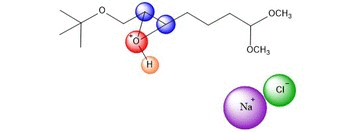
NH4Cl is in solution to act as a
mild acid. Protons are constantly being exchanged between the
ammonia and chloride ions in a similar manner to a buffer system.
The oxygen is then protonated, in the case we've shown, by the HCl.
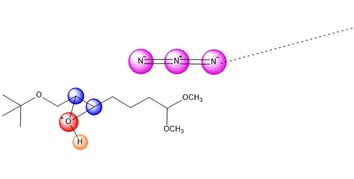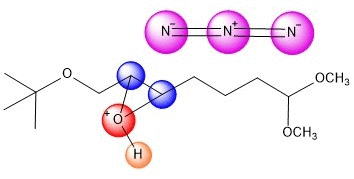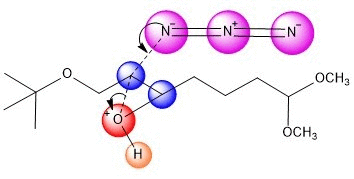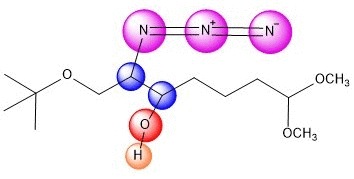
An azide ion, N3, in solution as NaN3
acts as a nucleophile to attack one side of the epoxide. It seems
plausible that tis regiochemistry would be more likely
to occur because of the electron-withdrawing group C=O.
At this point, the product is acheived, with NH3 and NaCl as by products