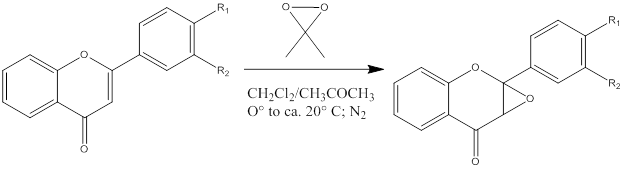1) Adam, W.; Golsch, D.; Hadjiarapoglou, L. Tetrahedron Letters 1991, 32, 1041-1044.
2) Lévai, A.; Jekő, J. Journal of Heterocyclic Chemistry 2004, 41, 439-443.
3) Burke, A. J.; O’Sullivan, W. I. Ttrahedron 1997, 53, 8491-8500.
4) Lévai, A.; Patonay, T.; Székely, A.; Vass, E. B.; Adam, W.; Jekö, J. Journal of Heterocyclic Chemistry 2000, 37, 1065-1069.
5) Adam, W.; Golsch, D.; Hadjiarapoglou, L. Tetrahedron Letters 1991, 32, 1041-1044.
6) Baumstark, A. L.; McCloskey, C. J. Tetrahedron Letters 1987, 28, 3311-3314.
7) Veloza, L. A.; Orozco, L. M.; Sepúlveda-Arias, J. C. Nat. Prod. Commun. 2011, 6, 925-930.
Citation relations to reaction:
1) Adam, W.; Golsch, D.; Hadjiarapoglou, L. Tetrahedron Letters 1991, 32, 1041-1044.
While not cited by our article, we had to choose this related article due to a lack of any reference materials. The conclusion of this paper is that while classical oxidants such as Hydrogen Peroxide, m-CPBA, SeO2, KMnO4, and NiO2 were difficult to use in epoxidation reactions of compounds like flavones, or in the case of our reaction, molecule SI-1, DMDO provided en easy and efficient way to form epoxides. DMDO is able to undergo a very efficient epoxidation reaction with both electron rich molecules like enol esters, as well as electron poor alkenes such as unsaturated acids with yields close to 100 percent. DMDO also is reactive and selective under strictly neutral conditions, which makes it ideal for avoiding proton transfers. These are all necessary qualities for our reaction, because for N3 to add in the second step correct selectivity, and ability to efficiently react with an electron poor unsaturated alkene in key. Without the correct selective reaction, the nitrogen will not add to the back, and the rest of the reaction will not lead to the correct pyrrole-surrogate that is part of the reaction scheme for Palau’amine.2) Lévai, A.; Jekő, J. Journal of Heterocyclic Chemistry 2004, 41, 439-443.
This article relates to the previous in that it is about using DMDO as an epoxidizing agent. The conclusion of the paper is that DMDO can be used successfully in the regioselective epoxidation of double bonds under neutral conditions. It also states that the reaction has a high yield and does not form any by-products.
3) Burke, A. J.; O’Sullivan, W. I. Ttrahedron 1997, 53, 8491-8500.
This article shows how different substituents around the site of epoxidation can undergo different reactions, as well as how regioselectivity is affected. The reaction scheme for our transformation from SI-1 to SI-2 is set, but if needed, information from this article could help modify the starting reagent to ensure a high yield based upon regioselectivity.
4) Lévai, A.; Patonay, T.; Székely, A.; Vass, E. B.; Adam, W.; Jekö, J. Journal of Heterocyclic Chemistry 2000, 37, 1065-1069.
This paper gives god information of the disadvantages of DMDO in specific cases of crowded reaction centers being to satirically hindered to allow for efficient high yield reactions with DMDO. While this is not a problem in our current mechanism, it is good to address all possible areas in our reaction where issues could occur, and have contingency plans to solve any problem.

.jpg)