Mechanism
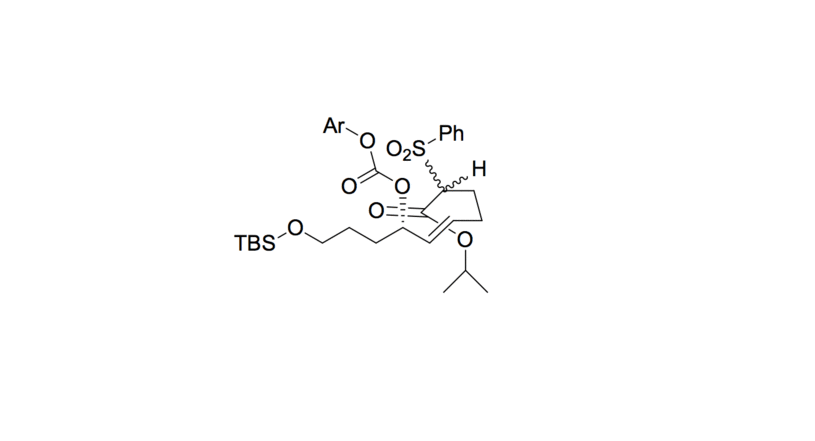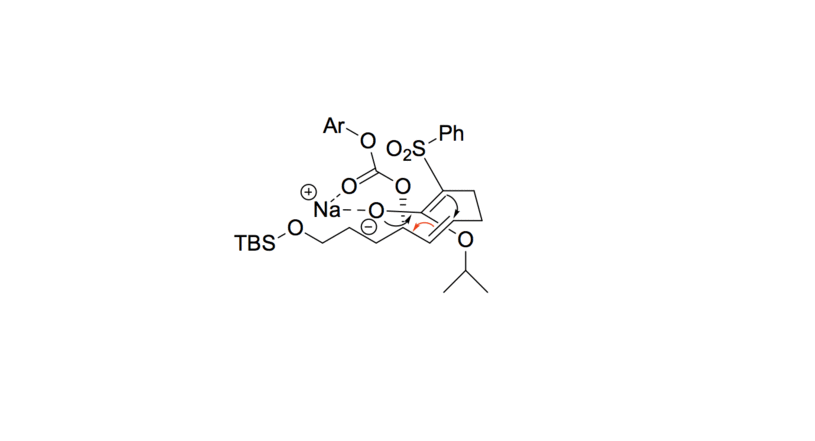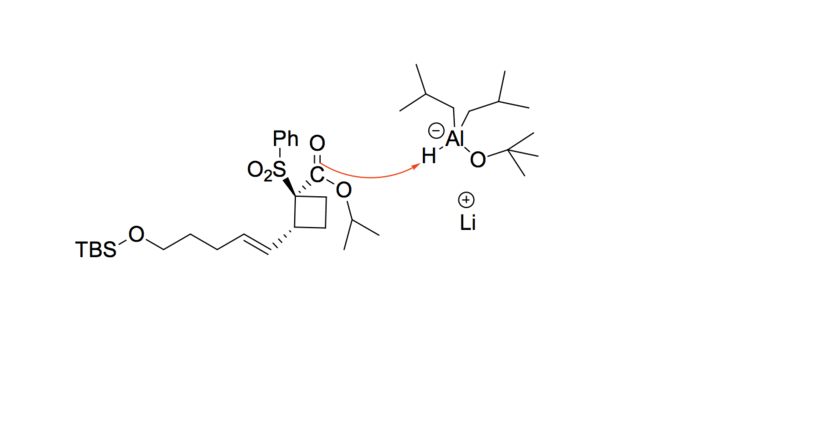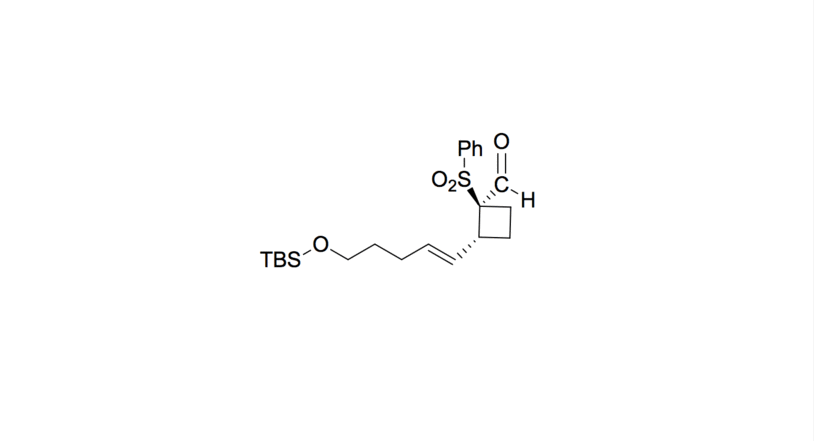
- The sulfonyl carbon is deprotonated, forming an enolate with the adjacent ester. The enolate oxygen anion is then stabilized by the sodium cation.
- The enolate undergoes conjugate addition with the alkene, forming a 4-member ring. The leaving group decomposes into CO 2 and an aryl alkoxide.
- A hydride ion adds to the carbonyl carbon, and the aluminum compound complexes with the oxygen anion, stabilizing it momentarily.
- The oxygen de-complexes from the aluminum compound and reforms the carbonyl, with the isopropyl alkoxide acting as a leaving group. The final aldehyde is formed.
