References
Main Reference
Kim, M. S.; Choi, Y.M.; An, D. K. Tetrahedron Lett. 2007, 48, 5061.
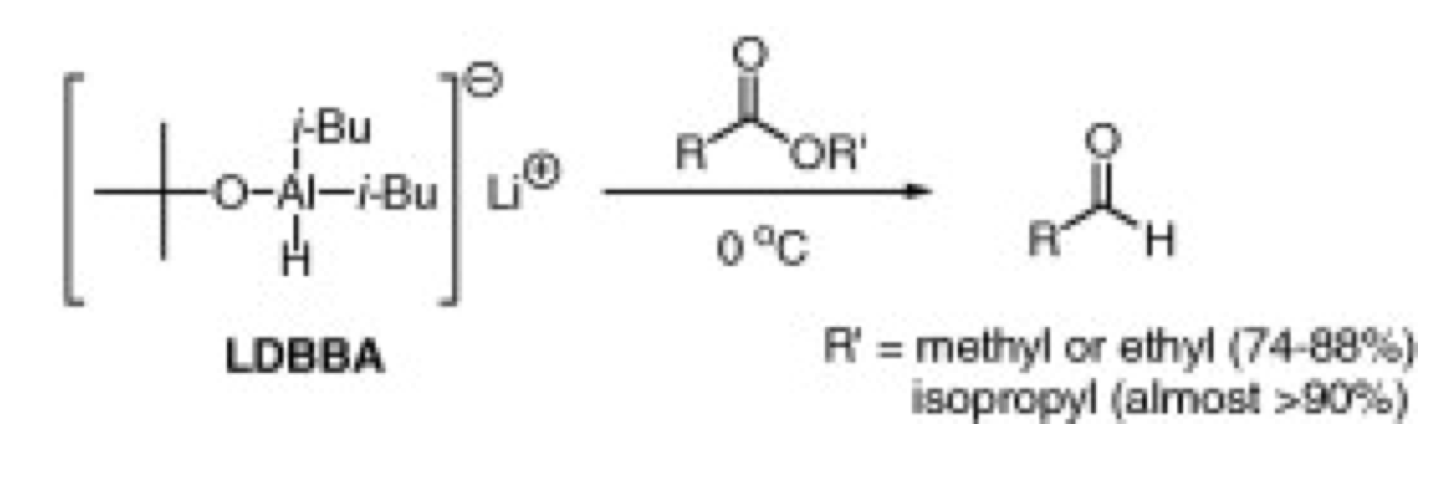
In the synthesis of molecule 14 as described in our assigned paper, lithium diisobutylalkoxyaluminum hydride is used to reduce purified cyclobutane of the molecule syn-13 to aldehyde 14. Lithium diisobutylalkoxyaluminum hydride was the most effective for the reduction of esters to aldehydes in good yield. Lithium diisobutylalkoxyaluminum hydride is a good reagent to use in this synthesis is because the reaction can be carried out at 0°C instead of very low temperatures or very high temperatures during reflux. The resulting aldehydes are produced in high yields. The isopropyl esters are more effective to produce aldehydes. Also, lithium diisobutylalkoxyaluminum hydride can reduce various aliphatic and aromatic isopropyl esters to aldehydes with a yield of greater than 90%. The researchers have shown that lithium diisobutylalkoxyaluminum hydride is an efficient alternative to reduce carboxylic esters to aldehydes. The researchers have shown that lithium diisobutylalkoxyaluminum hydride is an efficient alternative to reduce carboxylic esters to aldehydes.
Extra References
- Chae, M. J.; Jeon, A. R.; Livinghouse, T.; An, D. K. Chem. Commun. 2011, 47, 3281-3283
- Chae, M. J.; Song, J. I.; An, D. K. Bull. Korean Chem. Soc. 2007, 28, 2517-2518
Citing papers
- Boussonnière, A.; Bénéteau, R.; Lebreton, J.; Dénès, F. Eur. J. Org. Chem. 2013, 35, 7853–7866.
- Yuan, C.; Jin, Y.; Wilde, N. C.; Baran, P. S. Angew. Chem. Int. Ed. 2016, 55, 8280.
- An, J. S.; Shin, W. K.; An, D. K. Bull. Korean Chem. Soc. 2015, 36, 2928–2931.
Aluminum hydrides have become popular reagents to achieve the transformation of esters to aldehydes. The researchers used diisobutylaluminum hydride to a perform the partial reduction of esters to aldehydes in optimal reaction conditions to prevent an unstable aluminum acetal intermediate from undergoing a specific bond cleavage between a carbon atom and oxygen atom of an alkoxy group.
Diisobutylaluminum hydride was used as a reducing agent to stereo- and site- selectively produce an intermediate compound in the synthesis of the highly oxidized members of the Taxol family, which is used to treat of number of types of cancer and can be used to measure the progress in chemical synthesis.
The researchers of this article used LDBBA for the reduction of aromatic and aliphatic Weinreb amide to aldehydes for its reducing ability at 0°C and did not require longer reaction times