Leading Question: Stereoselectivity of SN2 reaction
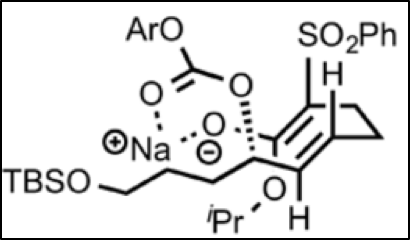
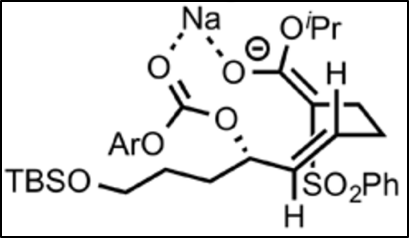
The SN2 reaction prefers the major pathway because of the bulky phenylsulphonyl group. This group prefers to be “up” instead of “down;” when down, it has significant steric interactions with the rigid olefinic (alkene) chain that is, more or less, bound in place by the sodium chelation. When up, the phenylsulphonyl group does not have as many steric interactions, and so it is more stable in the chelated form. Because the position of the phenylsulfonyl group determines the stereochemistry of the product after the ring closes, the preference of the phenylsulfonyl to be up determines that 11 is the major product, and 12 the minor.