Created by Susan Stagg-Williams, Dieter Andrew Schweiss, Gavin Sy, and H. Scott Fogler, 1994
Updated by Apeksha Bandi, Gustav Sandborgh, and Arthur Shih, 2013
1. Concentration of Venom in the Blood
The amount of venom in the blood would continuously decrease as it gets consumed in various reactions: adsorption on sites (reaction 1), reaction with antivenom molecules bound to the site (reaction 3), reaction with antivenom in the blood (reaction 5) and excretion from the body (reaction 7).

The overall rate of consumption of venom would be given by adding up the individual rates of consumption from all of these reactions.

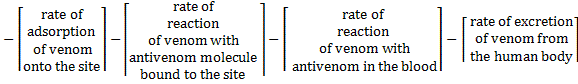

(E-5)
The terms on the right hand side are the rates of generation of venom (which will be negative since venom is being consumed and excreted) in reactions 1, 3, 5 and 7 respectively.

The rate equations for the elementary reactions are as follows:

(E-6)
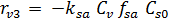
(E-7)
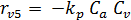
(E-8)
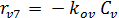
(E-9)
Combining the batch reactor design equation (equation E-4) with the material balance (equation E-5)and rate laws (equations E-6 to E-9) gives us the governing equation for concentration of venom in the blood:
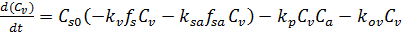
(E-10)
This is one governing differential equation of many. The complete governing equations for all the defined concentrations can be solved using POLYMATH by appropriate initial conditions to model the behavior.
Go Back