Chapter 2: Conversion and Reactor Sizing
Professional Reference Shelf
R2.1 Levenspiel Plots in Terms of Conversion
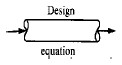
| For reactions in which the rate depends only on the concentration of one species [i.e., - rA = f(CA)], it is usually convenient to report -rA as a function of concentration rather than conversion. We can rewrite the design equation for a plug-flow reactor [Equation (R2.1-1)] in terms of the concentration, CA, rather than in terms of conversion for the special case when ν = ν0. | ||
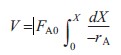 |
(R2.1-1) | |
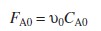 |
(R2.1-2) | |
| Also | ||
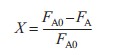 |
(R2.1-3) | |
| For the special case when ν=ν0, | ||
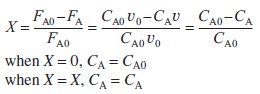 |
||
| Differentiating yields | ||
|
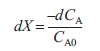 |
(R2.1-4) |
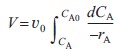 |
||
| Valid only if ν=ν0 | 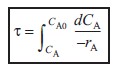 |
(R2.1-5) |
Equation (R2.1-5) is a form of the design equation for constant volumetric flow rate ν0 that may prove more useful in determining the space time or reactor volume for reaction rates that depend only on the concentration of one species. Figure R2.1-1 shows a typical curve of the reciprocal reaction rate as a function of concentration for an isothermal reaction carried out at constant volume. For reaction orders greater than zero, the rate decreases as concentration decreases. The area under the curve gives the space time necessary to reduce the concentration of A from CA0 to CA1. |
||
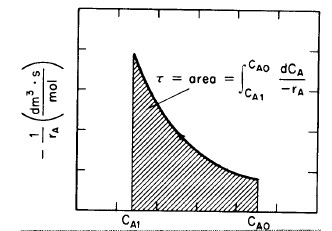 Figure 2.2-1 Determining the space time, τ. |
||