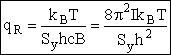Chapter 3: Rate Laws
Transition State Theory
Derivation of the Rotational Partition Function qR
Rigid Rotation 23
To show
| (R1) | ||||
| where | ||||
| (R2) | ||||
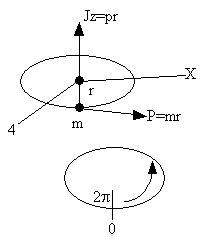
| Consider a particle of mass m rotating about the z axis a distance r from the origin. | ||||
| (R3) | ||||
 |
||||
| This time we convert the wave equation to spherical coordinate to obtain 24 | ||||
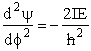 |
(R4) | |||
| Classical Energy of a rigid rotator is | ||||
| (R5) | ||||
| where w is the angular velocity (rod/s) and I is the moment of inertia 25 | ||||
| (R6) | ||||
| where mi is the mass located and distance ri from the center of mass. | ||||
| Quantum mechanics solutions to the
wave equation gives two quantum numbers, |
||||
| (R7)26 | ||||
| Let J ≡ |
||||
| For a linear rigid rotator | ||||
| (R8) | ||||
| Where B is the rotation constant: | ||||
| (R2)27 | ||||
| with | ||||
| The rotational partition function is | ||||
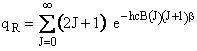 |
(R9)28 | |||
| Replacing the |
||||
|
(R10) | |||
| where Sy is the symmetry number which is the number of different but equivalent arrangements that can be made by rotating the molecules. | ||||
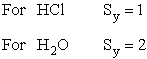 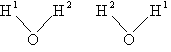 |
||||
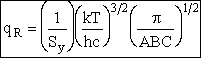 |
||||
| where |
||||
| Sy = symmetry number. 30 For a hetronuclear molecule σ= 1 and for a homonuclear diatomic molecule or a symmetrical linear molecule, e.g., H2, then σ= 2. | ||||
| Order of Magnitude and Representative Values | ||||
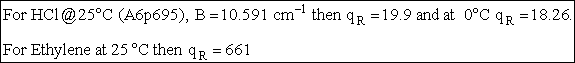 |
23P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), pp. 409, 413, 557, A24.
24P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), p. 410.
25P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), p. 555.
26P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), pp. 408, 413.
27P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), p. 557.
28P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), pp. 414, 563, 671.
29 P. W. Atkins, Physical Chemistry, 5th ed. (New York: Freeman, 1994), p. 694.
30For discussion of s, see K. J. Laidler, Chemical Kinetics, 3rd ed. (New York: Harper Collins, 1987), p.99.
Return toTransition State Theory