Chapter 5: Isothermal Reactor Design: Conversion
Additional Homework Problems
CDP6-28B
Ethyl acetate is an extensively used solvent and can be formed by the vapor-phase esterification of acetic acid and ethanol. The reaction was studied using a microporous resin as a catalyst in a packed bed reactor [Ind. Eng. Chem. Res., 26(2), 198 (1987)]. The reaction is first-order in ethanol and pseudo-first-order in acetic acid. For an equal molar feed rate of acetic acid and ethanol, the specific reaction rate is 1.2 dm3/ g cat / min. The total molar feed rate is 10 mol/min, the initial pressure is 10 atm, the temperature is 118 °C, and the pressure drop parameter, ![]() , equals 0.01 g-1.
, equals 0.01 g-1.
- Calculate the maximum weight of catalyst that one could use and maintain an exit pressure above 1 atm. (Ans: W = 99g)
- Determine the catalyst weight necessary to achieve 90% conversion. What is the ratio of catalyst needed to achieve the last 5% (85 to 90%) conversion to the weight necessary to achieve the first 5% (0 to 5%) conversion in the reactor? Vary
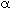 and write a few sentences describing and explaining your findings. What generalizations can you make? (Ans: W= 82g)
and write a few sentences describing and explaining your findings. What generalizations can you make? (Ans: W= 82g)
[3rd Ed. P4-18]