Chapter 5: Isothermal Reactor Design: Conversion
Additional Homework Problems
CDP6-31B
Alkylated cyclohexanols are important intermediates in the fragrance and perfume industry [ Ind. Eng. Chem. Re., 28, 693 (1989)]. Recent work has focused on gas-phase catalyzed hydrogenation of o-cresol to 2-methylcyclohexanone, which is then hydrogenated to 2-methylcyclohexanol. In this problem we focus only the first step in the reaction. The reaction on a nickle-silica catalyst was found to be zero-order in o-cresol and first order in hydrogen with a specific reaction rate at 170°C of 1.74 mol o-cresol/ (kg cat min atm). The reaction mixture enters the packed-bed reactor at a total pressure of 5 atm. The molar feed consists of 67% H2 and 33# o-cresol at a total molar flow rate of 40 mol/min.

- Neglecting pressure drop, plot the rate of reaction of o-cresol and the concentrations of each species as a function of catalyst weight. What is the ratio of catalyst weight needed to achieve the last 5% conversion to the weight necessary to achieve the first 5% conversion in the PFR.
- Accounting for pressure drop in the packed bed using a value of
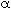 = 0.027 kg-1, redo part (a) along with a plot of pressure versus catalyst weight.
= 0.027 kg-1, redo part (a) along with a plot of pressure versus catalyst weight.
- Another engineer suggests that instead of changing catalyst size it would be better to pack the catalyst in a shorter reactor with twice the pipe diameter. If all other conditions remain the same, is this suggestion better?
[3rd Ed. P4-22]