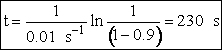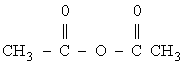Chapter 5: Isothermal Reactor Design: Conversion
Example 5.2: Home Problem
The hydrolysis of acetic anhydride is to be carried out at 25°C.
![]()
The water and acetic anhydride are mixed immediately before entering the reactor where the entering steam is 7.8 wt % acetic anhydride (1M) and 92.2 wt % water (51.2M). The volumetric feed rate of liquid in 0.0033 dm3/s. A one minute video clip of this experiment is given on the CDROM.
Additional Information
![]()
(a) What conversion can be achieved in a 1 dm3 CSTR at 25°C?
(b) What conversion can be achieved in a0.311 dm3 PFR?
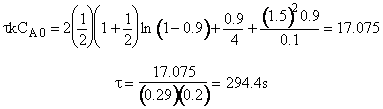
(c) What time is required to achieve 80% conversion in a batch reactor?
The 460 Experiment Hydrolysis of Acetic Anhydride (AH)
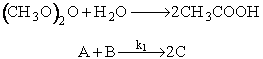

![]()
|
|
A |
|
|
|
|
|
B |
|
|
|
|
|
C |
0 |
|
|
(a) CSTR

1. Mole Balance
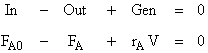
Rearranging
![]()
2. Rate Law
![]()
3. Stoichiometry liquid v = v0
![]()
![]()
![]()
![]()
![]()
![]()
4. Combine
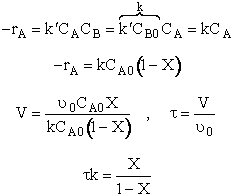
![]()
5. Evaluate
The maximum available flow rate in the experiment is ![]()
![]()
V = 1 dm3
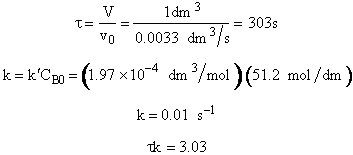
![]()
(b) PFR
![]()
1. Mole Balance/Design Equation
![]()
2. Rate Law
![]()
3. Stoichiometry
![]()
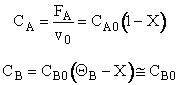
4. Combine


V = 0 X = 0
![]()
![]()
5. Evaluate

![]()
(c) Batch Reactor
1. Mole Balance/Design Equation
![]()
2. Rate Law
![]()
3. Stoichiometry
Liquid ![]()
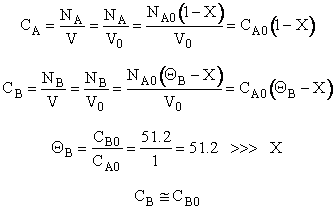
4. Combine


![]()
5. Evaluate