Chapter 5: Isothermal Reactor Design: Conversion
What Four Things are Wrong with this Solution?
Problem
The elementary gas phase reaction A + 2B --> C is carried out in a packed bed reactor. Calculate the exit conversion from a PBR packed with 800 kg of catalyst. The feed is stoichiometric.
Additional Information
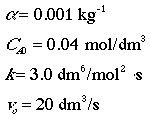
Solution
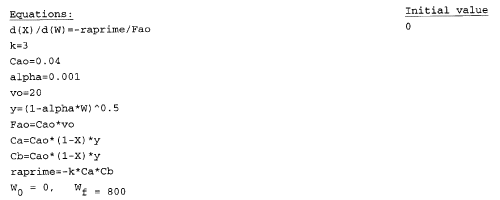
|
|
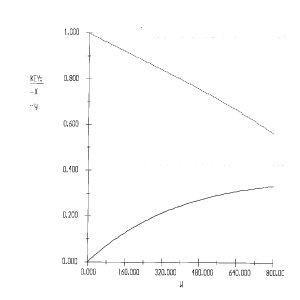
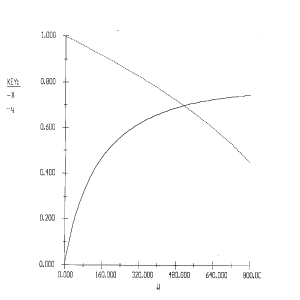
1) The rate law is incorrect. It should be: ![]()
2) The concentrations are incorrect, the feed is stoichiometric.
Therefore: ![]()
3) The volume change was not included.
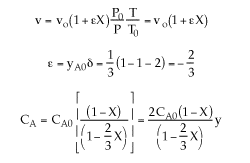
4) The equation for y is incorrect. There is volume change and ![]() can only be used when
can only be used when ![]() . Therefore, the form of the momentum balance that must be used is
. Therefore, the form of the momentum balance that must be used is 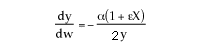
Corrected Solution