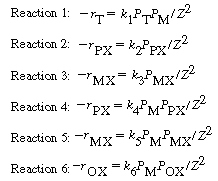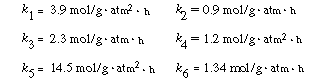Chapter 8: Multiple Reactions
Additional Homework Problems
CDP8-IC
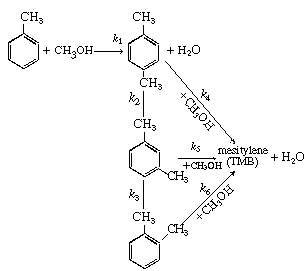
| The production of p -xylene is to be carried out by the reaction of toluene and methanol over HZSM-8 zeolite catalyst [Ind. Eng. Chem. Res. , 28, 890 (1989)]. The reaction sequence is | |||
|
|
|
||
| For the first approximation, we are going to neglect the reverse reactions. The rate laws are: | |||
|
|
|||
| where | |||
|
|
|||
| The subscripts used above are: T = toluene, M = methanol, PX = p -xylene, MX = m -xylene, OX = o -xylene, TMB = mesitylene. | |||
|
|
|||
|
The feed conditions are: P M = 5 atm, P T = 5 atm, T = 450°C, and the total molar feed rate is 10 mol/ min. (a) Which of the reactions can be neglected to further simplify
the kinetics? (1) Packed-bed reactor. (c) How would you go about maximizing (e.g., feed concentrations, ratio of toluene to methanol fed, etc.) the production of (1) p -xylene. (d) The activation energies for the six reactions are (in
kJ/mol): E1 = 60.5, E2 = 35.4, E3
= 45.9, E 4 = 35.8, |