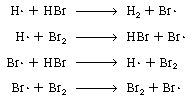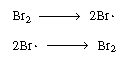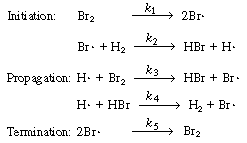Chapter 9: Reaction Mechanisms, Pathways, Bioreactions and Bioreactors
Learning Resources
Solved Problem - Hydrogen Bromide
| One of the most studied chain reaction is that between hydrogen and bromine: | |||
|
H2 + Br2 |
(CD7-1) | ||
| In studying this reaction we first deduce the rate law from reaction rate-concentration data and then formulate a mechanism and rate equation that are consistent with experimental observations. The relative rates of the reaction of bromine, hydrogen, and hydrogen bromide are | |||
|
|
(CD7-2) | ||
| Synthesis of the Rate Law from Experimental Data. An analysis of the experimental rate data shows the following observations. The reaction is: |
|||
|
Experimental |
|
||
|
Example CD7-1 |
|||
The Mechanism |
||
| We first postulate that the active intermediates are bromine
and hydrogen free radicals Br |
||
|
Propagation steps |
|
|
| The last reaction has no effect on the overall reaction because
one Br2and one Br |
||
|
|
||
| Note that our proposed scheme of reactions [Equations (CD7-3)
through (CD7-6)] produces one of the active intermediates in each step.
Now we only need a reaction yielding the initial formation of Br |
||
|
Initiation and termination steps |
|
|
|
|
||
|
|
As a first approximation we shall assume that the mechanism
consists of reactions (CD7-3), (CD7-4), and (CD7-6) through (CD7-8). If
this mechanism does not produce a rate equation that is consistent with
the experimental observations in Equation (CDE7-1.6), we could propose a
different mechanism that might include Equation (CD7-5) together with H2 |
|
|
|
||
|
The mechanism |
|
|
|
|
||
|
|
The specific reaction rates k1 and k5are defined with respect to Br2. | |
|
|
Example CD7-2 |
|